PART 3: Experiments to prove things about Respiration
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
What is the procedure for the experiment to show that foods release energy when they are oxidized
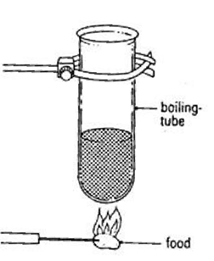
Measure 20 cm³ of water and pour it into a boiling tube.
Clamp the tube to a stand and record the starting temperature of the water.
Weigh a small piece of food (e.g. peanut) and write down its mass.
Stick the food onto a mounted needle.
Light the food using a Bunsen burner.
As soon as it catches fire, hold it under the boiling tube (without touching the tube).
Let the food burn completely while heating the water.
When the flame goes out, record the highest temperature of the water.
Calculate the temperature change.
Use the formula to calculate energy released:
Energy released (J) = mass of food (g) x temp rise (C) x 4.2/ mass of water (g)
What does this experiment prove
that food (glucose) releases energy when it is oxidized just like what happens in cells during respiration
Why is this experiment not super accurate
Not all the heat from the burning food goes to the water, some escapes to the air
The boiling tube loses some heat to its surroundings
The flame may not stay directly under the boiling tube or be close enough, so less heat reaches the water
To sum up: due to many heat losses
What precautions could you take to try to carry out a more accurate experiment
try to keep the food directly under the boiling tube
stir the water before taking initial temperature (ensures heat is evenly distributed)
What does this experiment show
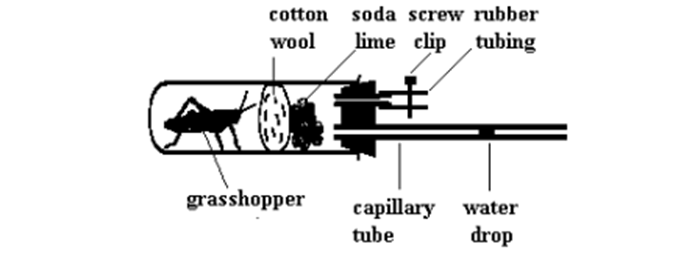
It shows that oxygen is used up during respiration
What significance do these things have in this experiment?
grasshopper
soda lime
cotton wool
the capillary tube with the water drop
grasshopper: is a living organism and therefore respires
soda lime: absorbs the carbon dioxide the grasshopper breathes out and prevents it from suffocating (build up of CO2)
cotton wool: protects the grasshopper from the soda lime since its corrosive
water drop: shows the movement of air towards the organism which shows that the organism is taking up air and hence oxygen
What should you do before starting this experiment?
Allow the grasshopper to acclamatise (get used to its conditions)
How could you prove this experiment actually works (results are caused only because of the grasshopper)
You could set up a control using the same apparatus, but not the living organism
Why may the bubble still move in the control
because the soda lime will absorb carbon dioxide in the air in the boiling tube
(however the movement of the bubble should be less than that with the organism and should stop after some time)
What does this experiment show
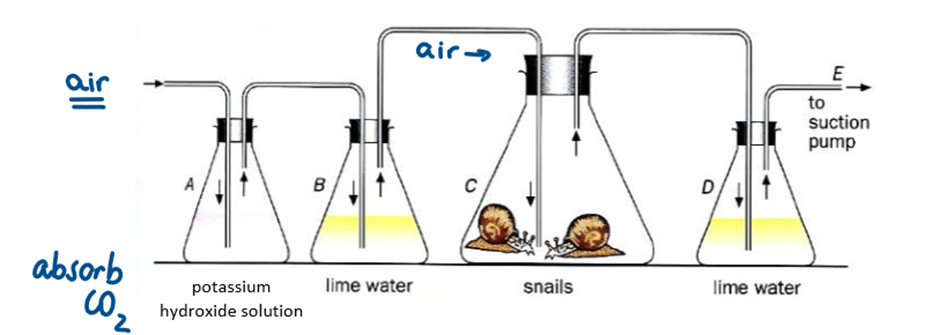
It shows that carbon dioxide is produced during respiration
Why was the air first bubbled in potassium hydroxide solution
because it absorbs the carbon dioxide from the air
Why was the air then bubbled in limewater (or hydrogen carbonate solution) before reaching the snails
to ensure there was no carbon dioxide left in the air, and it had all been absorbed by the potassium hydroxide solution
Which conical flask would have changed colour?
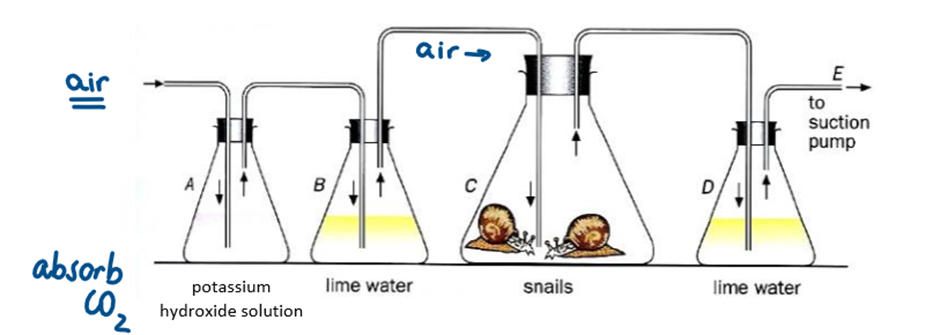
Flask D - turned milky-white showing that snails released carbon dioxide during respiration
What is the difference between limewater and hydrogen carbonate indicator
They both test for carbon dioxide
Limewater → Clear to milky-white
Hydrogen carbonate indicator: Red → Yellow
What does this experiment show
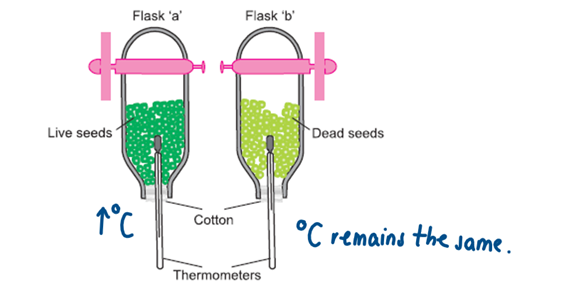
That heat is produced during respiration
Two identical vacuum flasks are half filled with germinating live peas (A) and dead peas (B) (boiled peas) which have soaked for 24 hours and rinsed in dilute disinfectant for about 5 minutes.
The apparatus is set up as shown in the diagram above.
The temperature of both flasks is recorded at the start and end of the experiment.
Which flask should show the higher temperature?
Flask A - live seeds will respire and therefore give off heat
why have the peas ‘been soaked for 24 hours’ beforehand
To start germination and therefore respiration (dry peas are dormant and don’t respire much)
Why were the peas ‘rinsed in dilute disinfectant’
To prevent any fungal or bacterial growth which will respire (and give off heat) affecting the accuracy of the experiment
Why should the flasks not be completely filled with peas
To allow air to circulate and prevent overheating (to not kill the peas)
Why are the flasks left upside down with porous cotton plugs
Because carbon dioxide is a denser gas than air and if the flask was upright and the plug didn’t allow carbon dioxide to escape, it would stay trapped in there and build up which could affect the results or harm the peas
Is all the energy produced by the peas during respiration given off as heat
No, some of it will be used by the peas to survive and carry out the 7 vital functions
What does this experiment show
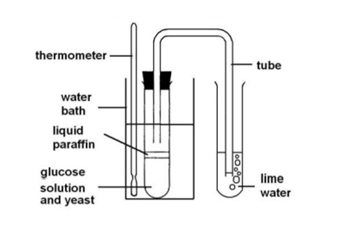
That carbon dioxide is produced when yeast respires anaerobically
In this experiment why is:
the water boiled
some sugar added to the water
why glucose solution was boiled
the liquid allowed to cool before adding the yeast
liquid paraffin/ oil added
boiling water: to remove any dissolved air
sugar added: needed for the yeast to respire
boil glucose solution: to kill any microorganisms that will respire
allowed to cool: yeast die at high temperatures
liquid paraffin/oil: blocks oxygen (ensures yeast respires anaerobically)
What should happen to the limewater/hydrogen carbonate indicator
Limewater - clear → milky white
Hydrogen carbonate indicator - red → yellow
This shows carbon dioxide has been released
How can you ensure these results are due to the anaerobic respiration of the yeast
Set up a control whereby the yeast is killed