rate & extent of chemical change topic 6
1/16
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
whats rate of chemical reaction, slow reactions example, another slow example & explain, moderate speed example, 2 fast examples
rate of chemical reaction → how fast reactants r changed into products
one of slowest → rusting of iron
other slow reactions → chemical weathering (acid rain damage to limestone buildings)
moderate speed → metal magnesium reacting w acid to produce gentle stream bubbles
fast → burning, explosions r even faster & release lots gas all over in fraction of second
what does collision theory, what 2 things rate of chemical reaction depends on, whats activation energy
reaction rate is explained by collision theory
rate of chemical reaction depends on:
collision frequency of reacting particles (how often collide), more collisions = faster reaction
energy transferred during collision, particles have to collide w enough energy for collision to be successful
minimum amount of energy particles need to react → activation energy (need this much energy to break bonds in reactants & start reaction
4 things rate of reaction depends on
temperature
concentration of solution/ pressure of gas
surface area - changes depending on size of lumps of solid
presence of catalyst
how does temp, concentration/ pressure, SA, catalyst increase the rate
increasing temp increases rate:
when temp increases = particles move faster
moving faster = collide more frequently
also move faster = more energy they have = more collision will have enough energy make reaction happen
increasing concentration/ pressure increases rate:
solution made more concentrated = more particles knocking about in same volume of water/ other solvent
pressure of gas increases = same no. particles occupy smaller space
this makes collisions between reactant particles more frequent
increasing SA increases rate:
one of reactants is solid, breaking it up into smaller pieces will increase its SA:V ratio
means for same volume of solid particles around it have more area to work on → Collisions happen more frequently
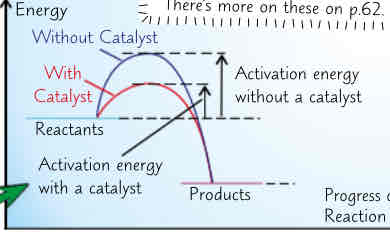
using catalyst increases rate:
catalyst → substance that speeds up reaction, w/out being used up itself (not apart of overall reaction equation)
diff catalysts r needed for diff reactions but all work by decreasing activation energy needed for reaction to occur → provide alternative reaction pathway w lower activation energy
enzymes = biological catalysts (catalyse reactions of living things)
equation for rate of reaction, what r the units for gas, solid & time
rate of reaction = amount of reactants or amount of product formed / time
gas = cm³, moles
solid = grams, moles
time = s
how can we measure rate of reaction using precipitation & colour change, change in mass, volume of gas given off
precipitation & colour change:
can record visual changes in reaction if initial solution is transparent & product is a precipitate which clouds solution (becomes opaque)
can observe mark through solution & measure how long it takes for it to disappear
if reactants r coloured & products r colourless (vice versa), can time how long it takes for solution to lose/ gain its colour
cant plot rate of reaction graph from results
change in mass (usually gas given off):
measuring speed of reaction that produces gas can be carried out using mass balance
gas released = mass disappearing is measured on balance
take measurements at regular intervals, can plot rate of reaction graph & find rate quite easily
most accurate of 3 methods because mass balance is very accurate but has disadvantages of releasing gas straight into room
volume of gas given off:
involves using gas syringe to measure volume of gas given off
more gas given off during given time interval = faster reaction
gas syringes usually give volume accurate to nearest cm³ = quite accurate, take measurements at regular intervals & plot rate of reaction graph but have to be careful → if reaction is too vigorous can easily blow plunger out of end of syringe
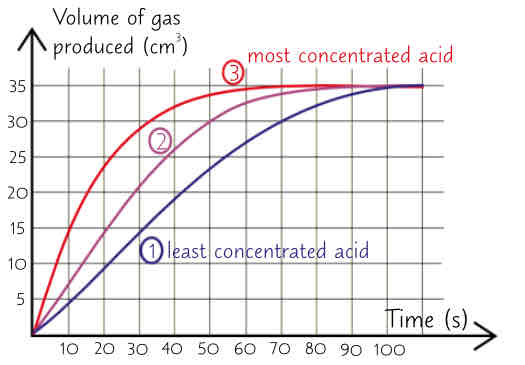
how to measure rate of reaction using magnesium & HCl which react to produce H_2 gas (6 steps)
add set volume of dilute hydrochloric acid to a conical flask
add some magnesium ribbon to acid & quickly attach empty gas syringe to flask
start stopwatch. Take readings of volume of gas in gas syringe at regular intervals, recording results in table
plot graph w time on x-axis & volume of gas on y-axis
repeat w more concentrated acid solutions. variables such as amount of magnesium ribbon & volume of acid used should be kept same each time - only change acids concentration to make experiment fair test
3 graphs show higher concentration of acid gives faster rate of reaction
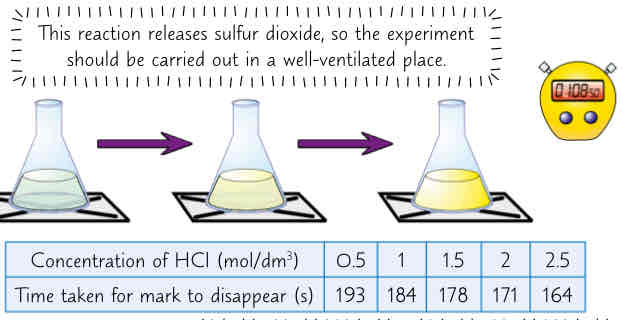
how to measure rate of reaction of sodium thiosulfate & HCl which produce a cloudy precipitate (8 steps)
these 2 chemicals r both clear solutions. React tgther to form yellow precipitate of sulfur
add set volume of dilute sodium thiosulfate to conical flask
place flask on piece of paper w black cross drawn on it. Add some HCl to flask & start stopwatch
watch black cross disappear through cloudy sulfur & time how long it takes
reactants should be replaced w solutions of either reactant at diff concentrations (only change 1 at a time). Depth of liquid must be kept same each time
results show effect of increasing concentration of HCl on rate of reaction, when added to excess of sodium thiosulfate
higher concentration = quicker reaction & therefore less time takes for mark to disappear
doesnt give set of graphs
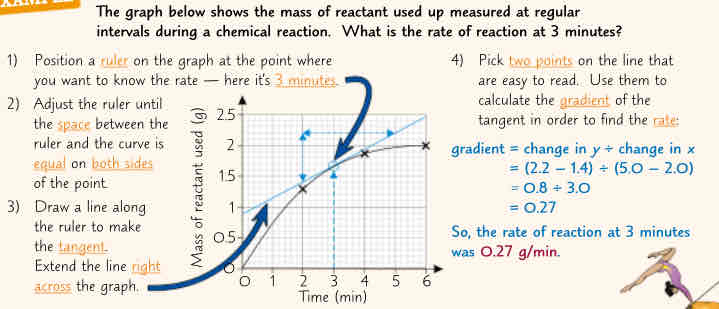
hows a rate of reaction graph shown, how to find mean rate for whole reaction, find mean rate of reaction between 2 points
rate of reaction graph show amount of product formed or amount of reactant used uo on y-axis & time on x-axis
find mean rate for whole reaction, just work out overall change in y-value & divide this by total time taken for reaction
can also use graph to find mean rate of reaction between any 2 points in time:
mean rate of reaction = change in y/ change in x

how to find reaction time at particular point
…
whats reversible reaction, as reactants react…, hows a system at equilibrium, at equilibrium…, equilibrium can only be reached…
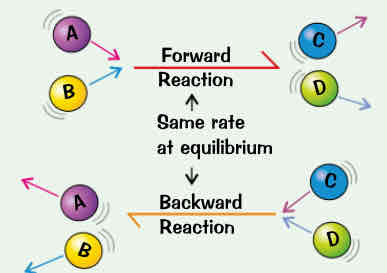
reversible reaction → products can react to form reactants again:
as reactants react their concentrations fall so forward reaction will slow down but as more products r made & their concentrations rise the backward reaction will speed up
after while forward reaction will be going at exactly same rate as backward one → system is at equilibrium
at equilibrium → both reactions r still happening but theres no overall effect (dynamic equilibrium) = concentrations of reactants & products have reached balance & wont change
equilibrium only reached if reaction takes place inside closed system (non of reactants or products can escape & nothing can get in)
when a reactants at equilibrium…, equilibrium lies to right…, to left…, 3 things position of equilibrium depends on
when a reactants at equilibrium it doesnt mean amounts of reactants & products r equal
equilibrium lies to right = concentration of products is greater than that of reactants
equilibrium lies to left = concentration of reactants is greater
position of equilibrium depend on:
temp → heating reaction moves equilibrium to right & cooling to left
pressure (only affects equilibrium involving gases)
concentration of reactants & products
in reversible reactions…, energy transferred from surroundings by endothermic = …, give example using blue hydrated copper (II) sulfate crystals
in reversible reactions if reaction is endothermic then it will be exothermic in other direction
energy transferred from surroundings by endothermic reaction = energy transferred to surroundings during exothermic reaction
example: heat blue hydrated copper (II) sulfate crystals, it drives water off & leaves white anhydrous copper (II) sulfate powder → endothermic
add couple drops of water to white powder get blue crystals back → exothermic
whats Le Chatelier’s principle, what can it be used for
Le chatelier’s principle → idea if change conditions of reversible reaction at equilibrium, the system will try to counteract that change
can be used to predict effect of any changes make to reaction system
what are all reactions, decrease temp.., raise temp.., (involves exothermic & endothermic & equilibrium)
all reactions r exothermic & endothermic in each direction
decrease temp = equilibrium moves in exothermic direction to produce heat → get more products for exothermic reaction & fewer for endothermic reaction
raise temp = equilibrium moves in endothermic direction to try & decrease it → get more products for endothermic reaction & fewer products for exothermic reaction

changing pressure affects…, increasing pressure.., decreasing pressure…, what can use a balanced equation for
changing pressure only affects equilibrium involving gases
increase pressure → equilibrium tries to reduce it = moves in direction where there r fewer molecules of gas
decrease pressure → equilibrium tries increase it = moves in direction where there r more molecules of gas
can use balanced symbol equation for reaction to see which side has more molecules of gas

changing concentration…, system responds…, increasing concentration of reactants…, decreasing concentration of products..
change concentration of either reactants/ products the system will no longer be at equilibrium
system responds to bring itself back to equilibrium again
increase concentration of reactants→ system tries to decrease it by making more products
decrease concentration of products → system tries to increase it again by reducing amount of reactants
