HAP22306 Theory week 2
0.0(0)
0.0(0)
Card Sorting
1/117
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
118 Terms
1
New cards
What is the use of Fab fragments in immunochemical applications?
Fab fragments can be useful for some immunochemical applications where the larger native antibody molecule would have difficulty binding.
2
New cards
* What are the three fragments generated by enzymatic digestion of antibodies using papain?
\
\
Enzymatic digestion of antibodies using papain generates three fragments: two antigen-binding fragments (Fab) and one constant fragment (Fc), with the Fab fragments retaining their antigen-binding capability.
3
New cards
* What is the antigen-binding site of an antibody?
\
\
The variable regions of both heavy and light chains form the antigen-binding site, which defines the specificity of the antibody.
4
New cards
* What is the difference between the constant and variable region of antibodies?
\
\
The constant region of the antibody is conserved within a species, while the variable region varies greatly among different antibodies.
5
New cards
Magnetic latex particles
Latex particles with magnetic properties used for easy separation from liquid phase
6
New cards
Protein A
A protein that can be covalently attached to the surface of latex and magnetic particles to maximize the chance of successful encounter with the antigen-binding site.
7
New cards
Light microscope
Microscopes that use glass lenses to focus light on a specimen in order to form an image, with a maximum magnification of around 1500 times.
8
New cards
Electron microscope
Microscopes that use electromagnetic lenses to focus a beam of electrons on a specimen, with a maximum magnification of approximately 200,000 times.
9
New cards
Resolution
The ability to distinguish between two closely spaced points in a specimen, which is a more reliable estimate of a microscope's usefulness than magnification.
10
New cards
Lateral resolution
The ability to distinguish between two closely spaced points in the x and y directions of a specimen.
11
New cards
Super resolution microscopes
Specialized instruments that can improve the theoretical maximum resolution limit of the light microscope to 0.1 micrometers.
12
New cards
Biomedical research
Research aimed at improving human health and curing diseases, which can involve the use of microscopes to study a wide range of specimens.
13
New cards
Living specimens
Specimens that are still alive and require different microscopy techniques, such as time-lapse imaging, to study their behavior and changes.
14
New cards
Non-living specimens
Specimens that are no longer alive and are suitable for electron microscopy, which provides higher resolution but cannot be used to observe living cells.
15
New cards
Magnification
The ability to make a specimen appear larger, which is limited by the resolution of the microscope.
16
New cards
Compound light microscope
A microscope composed of several main components, including a light source, a condenser lens, an objective lens, and an eyepiece lens. The image is magnified and brought into focus by the objective and eyepiece lenses, and can be viewed directly or projected onto a detector such as photographic film or a digital camera.
17
New cards
Light source
The component of a compound light microscope that provides illumination to the specimen, which can be a mercury or xenon lamp, laser, or LED.
18
New cards
Condenser lens
The lens of a compound light microscope that focuses the light source onto the specimen and produces uniformly bright and glare-free illumination across the specimen's viewing area if correctly positioned (Köhler illumination).
19
New cards
Objective lens
The lens of a compound light microscope that produces the magnified image of the specimen and can be the most expensive component of a light microscope.
20
New cards
Numerical apperature
A measure of the objective lens's ability to collect light from the specimen and always marked on the lens. Lenses with a high NA have better resolution than those with a low NA.
21
New cards
Resolution
A measure of the ability of a lens to distinguish between two objects in the specimen. The NA and magnification of the objective lens affect the resolution, with higher NA and magnification producing better resolution.
22
New cards
Stand
The component of a compound light microscope that holds all of the other components in place. There are two basic types - an upright and an inverted stand.
23
New cards
Illumination
The correct illumination of the specimen is important for high-quality images in microscopy, and light sources such as mercury or xenon lamps, lasers, or LEDs are commonly used. The light passes through the condenser lens and can be adjusted for focusing.
24
New cards
Stage
The component of a compound light microscope that holds the specimen firmly in place and enables it to be moved and positioned in fine and smooth increments both horizontally and transversely for locating a region of interest.
25
New cards
Coverslip
\
\
A thin glass or plastic slip that is placed over the specimen on the microscope slide to protect it and keep it in place.
26
New cards
What is bright-field illumination and when is it useful?
Bright-field illumination is the most basic mode of light microscopy and requires a minimum of optical elements. Contrast in bright-field images is usually produced by the color of the specimen itself, making it useful for pigmented tissues, histological sections, or tissue culture cells stained with colorful dyes.
27
New cards
What is tissue processing?
Procedure to remove water from cells and replace with a medium (paraffin or resin) to solidify tissue, allowing cutting of thin sections.
28
New cards
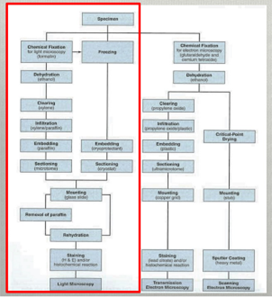
Steps of tissue preparation
1) Fixation
2) Dehydration
3) Clearing
4) Infiltration
5) Embedding
6) Sectioning
7) Mounting
8) Rehydration
9) Staining
2) Dehydration
3) Clearing
4) Infiltration
5) Embedding
6) Sectioning
7) Mounting
8) Rehydration
9) Staining
29
New cards
Why do we prefer chemical fixation often over freezing?
You can also freeze tissue instead of fixating. However, with fixating (with paraffin) you give the tissue structure, and by freezing the tissue it will be pressed together. E.g. ovaries are better fixed than frozen to see the oocytes nicely.
30
New cards
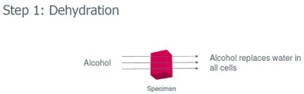
Tissue dehydration
* Paraffin is hydrophobic, so water needs to be removed
* Alcohol progressively replaces water in cells (gradient from 50% to 100%)
\
31
New cards
Tissue clearing
* Alcohol and paraffin are not miscible, thus an intermediate solvent needs to be used, such as xylene that displaces the alcohol
* Clearing, tissue becomes more transparent
* Removal of fat from tissue by this method could affect infiltration of paraffin.
* Clearing, tissue becomes more transparent
* Removal of fat from tissue by this method could affect infiltration of paraffin.
32
New cards
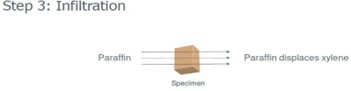
Tissue infiltration
* Now tissue can be infiltrated with paraffin
* Paraffin provides structural support to tissue (important for cutting!!)
* Paraffin provides structural support to tissue (important for cutting!!)
33
New cards
Tissue Trek system
consists of two modules that together create an ergonomic workstation for the production of paraffin blocks. The user can position the individual modules from either left to right or right to left, depending on personal preference.
* Using a tissue trek system the tissue can now be further processed
* Cooling down of paraffin
* Using a tissue trek system the tissue can now be further processed
* Cooling down of paraffin

34
New cards
Tissue cutting
* If sections are very wide, the paraffin block can be trimmed to facilitate the cutting of good sections
Cutting of paraffin sections:
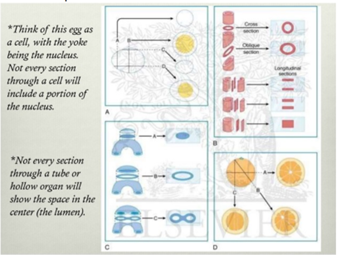
* Angle of sectioning is important for what you will see later
Cutting of paraffin sections:
* Angle of sectioning is important for what you will see later
35
New cards
General staining procedure
* First paraffin needs to be removed from tissue, otherwise staining will not work! à Done by e.g. Xylene, then an alcohol dilution and water.
* Staining
* Dehydration and mounting → prefer Xylene over water because of preservation.
* Staining
* Dehydration and mounting → prefer Xylene over water because of preservation.
36
New cards
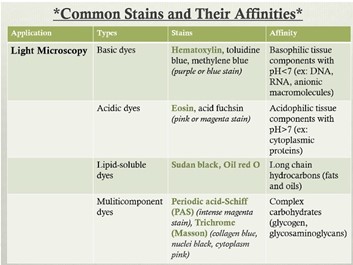
General stains
* (Chemical) characteristics of the tissue affects staining. à e.g. Masson trichrome stains collagen. Crossmon stains glycogen and connective tissue.
* So use the dye dependent on what you want to see.
* So use the dye dependent on what you want to see.
37
New cards
Staining of fat
* Freeze + oil binding stain à does give a less preserved structure
* Or stain with Sudan Black
* Non-fixed when you want quantitative info about the amount of fat.
* Or stain with Sudan Black
* Non-fixed when you want quantitative info about the amount of fat.
38
New cards
Antibodies and immunohistochemistry:
Large numbers of antibodies available, but:
o How specific are these antibodies?
o What are the proper controls to perform?
Antibodies are handy to get to know the function of a tissue or if a gene is being transcribed.
o How specific are these antibodies?
o What are the proper controls to perform?
Antibodies are handy to get to know the function of a tissue or if a gene is being transcribed.
39
New cards
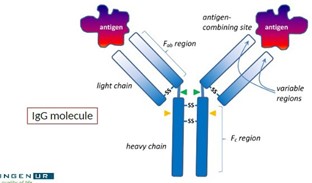
What are antibodies?
* Several types, most common being IgG
* Produced by B cells (plasma cells) upon infection with a specific antigen
* IgG contains specific antigen recognition site (antigen binding site)
* Produced by B cells (plasma cells) upon infection with a specific antigen
* IgG contains specific antigen recognition site (antigen binding site)
40
New cards
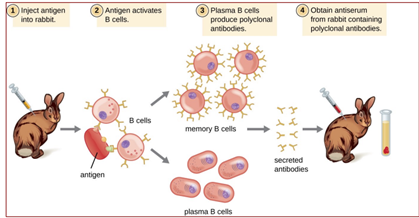
Polyclonal antibodies
* Immunization with large antigen – so-called polyclonal antisera – many different IgGs generated each recognizing a different isotope of the antigen.
* Further purification IgG fraction: affinity-purified antisera, enriched in a specific IgG, but still binding antigen at multiple sites.
* Raised in rabbits, guinea pigs, donkeys, goats, sheep; occasionally rats, chickens.
* Further purification IgG fraction: affinity-purified antisera, enriched in a specific IgG, but still binding antigen at multiple sites.
* Raised in rabbits, guinea pigs, donkeys, goats, sheep; occasionally rats, chickens.
41
New cards
Monoclonal antibodies
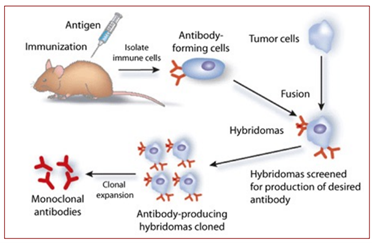
* Immunization of living mice (sometimes rats or rabbits)
* Titer of specific polyclonal antibody sufficient-> mouse killed, spleen removed and individual antibody-producing lymphocytes (producing different individual (cell)-specific IgGs) are immortalized.
* Production of clonal cell lines (hybridoma’s) capable of making specific IgGs; each clone secreting one specific monoclonal antibody in the medium
* Other Ig types may be generated – IgA, IgD, IgE and IgM.
* Titer of specific polyclonal antibody sufficient-> mouse killed, spleen removed and individual antibody-producing lymphocytes (producing different individual (cell)-specific IgGs) are immortalized.
* Production of clonal cell lines (hybridoma’s) capable of making specific IgGs; each clone secreting one specific monoclonal antibody in the medium
* Other Ig types may be generated – IgA, IgD, IgE and IgM.
42
New cards
What is a hybridoma and its advantages?
* Hybridoma = infinite source of antibodies by subculturing and freezing those cells.
* Advantage of hybridomas = always the same quality of antibodies.
* Advantage of hybridomas = always the same quality of antibodies.
43
New cards
Advantages polyclonal antibodies
* Recognize multiple epitopes on one antigen
* Heterogeneous mixture of different IgGs
* Bind with higher affinity, more tolerant to antigen variation due to for instance fixation
* More stable over a broad pH and salt concentration
* Heterogeneous mixture of different IgGs
* Bind with higher affinity, more tolerant to antigen variation due to for instance fixation
* More stable over a broad pH and salt concentration
44
New cards
Disadvantages polyclonal antibodies
* Greater likelihood of cross-reactivity
* Risk of high background
* Batch-to-batch variability (lot numbers), rabbit may have died
* Risk of high background
* Batch-to-batch variability (lot numbers), rabbit may have died
45
New cards
Advantages monoclonal antibodies
* Hybridoma constant renewable source of identical antibodies
* Increased reproducibility
* Single specific Igg means lower background
* Increased reproducibility
* Single specific Igg means lower background
46
New cards
Disadvantages monoclonal antibodies
* Often low concentration (high dilution) but modest affinity
* High epitope specificity, less tolerant to antigen variation/damage
* High epitope specificity, less tolerant to antigen variation/damage
47
New cards
Monotypic antibodies
Monotypic antibodies raised against a synthetic peptide of 10-14 amino acids, representing a single anti-genetic epitope, though polyclonal in terms of production
48
New cards
Advantages monotypic antibodies
* Simple production procedure
* Uses small peptides as immunogens
* Can tailor antibodies to specific epitopes
* Uses small peptides as immunogens
* Can tailor antibodies to specific epitopes
49
New cards
Disadvantages monotypic antibodies
* Single epitope recognition thus lower affinity
* Short peptide sequences are not strongly anti-genetic, thus in production process linked to larger (carrier) protein
* High background and cross-reactivity
* Short peptide sequences are not strongly anti-genetic, thus in production process linked to larger (carrier) protein
* High background and cross-reactivity
50
New cards
What is immunohistochemistry?
It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of (fluorescent) antibodies binding specifically to antigens in biological tissues.\[1\] IHC takes its name from the roots "immuno", in reference to antibodies used in the procedure, and "histo", meaning tissue
![It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of (fluorescent) antibodies binding specifically to antigens in biological tissues.\[1\] IHC takes its name from the roots "immuno", in reference to antibodies used in the procedure, and "histo", meaning tissue](https://knowt-user-attachments.s3.amazonaws.com/b6eb072a0fa74138aad64bbc9e06d640.jpeg)
51
New cards
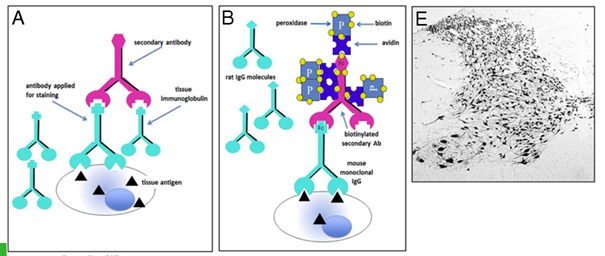
The avidin-biotin peroxidase (ABC) approach
The ABC method is a three-step detection method: the primary antibody binds to the antigen in the tissue; it is in turn recognized by the biotinylated secondary antibody; complexes formed by avidin and biotinylated enzyme (ABC) can then attach to the latter. Since the avidin tetramer can bind four biotinylated biotins, large ABC lattices containing several reporter enzymes are formed, leading to a strong amplification of the signal at the antigenic site.
* Amplification of weak signals, one avidin molecule can bind to multiple biotin molecuels
* Higher dilution of primary antibody (higher specificity)
52
New cards
Validating antibodies with controls:
Omission of primary antiserum: → this is NOT the proper control for specificity
* Control for non-specific binding of the secondary antibody!
* Says little about specificity primary antibody
Substitution primary antibody by equivalent amount (pre-immune) serum
* Pre-immune serum not always available, or expensive and can only be used for this specific antibody
* Normal serum from the same species primary antibody was raised
Replacement of primary antibody by an isotype IgG:
* Determines whether primary antibody IgG sticks to the section
Pre-absorption of antibodies using immunizing antigen or equivalent:
Antibody solution preincubated with the immunizing antigen (peptide/protein) in considerable molar excess:
* Quenching any available specific sites of the IgG for the target protein in the tissue section
* Does not exclude the possibility that antibodies are recognizing so-called non-specific epitopes in target tissue.
* Control for non-specific binding of the secondary antibody!
* Says little about specificity primary antibody
Substitution primary antibody by equivalent amount (pre-immune) serum
* Pre-immune serum not always available, or expensive and can only be used for this specific antibody
* Normal serum from the same species primary antibody was raised
Replacement of primary antibody by an isotype IgG:
* Determines whether primary antibody IgG sticks to the section
Pre-absorption of antibodies using immunizing antigen or equivalent:
Antibody solution preincubated with the immunizing antigen (peptide/protein) in considerable molar excess:
* Quenching any available specific sites of the IgG for the target protein in the tissue section
* Does not exclude the possibility that antibodies are recognizing so-called non-specific epitopes in target tissue.
53
New cards
Does the perfect antibody control exist?
The perfect control does not exist!!! Use literature and common sense à is this protein/receptor present in your tissue?
54
New cards
Why use immunofluorescence?
\
\
We use immunofluorescence to have the possibility to detect two or more antigens within one cell.
* Sensitive
* With correct controls and programs quantification of signal possible
* Sensitive
* With correct controls and programs quantification of signal possible
55
New cards
Issues related to immunofluorescence:
\
\
* Quality and choice of suitable fluorophores and corresponding excitation and emission filters
* Essential to control individually for each fluorophore being used, to ensure that chosen wavelengths for the excitation and emission filters are indeed mutually discrete, especially in case of double labelling
* Essential to control individually for each fluorophore being used, to ensure that chosen wavelengths for the excitation and emission filters are indeed mutually discrete, especially in case of double labelling
56
New cards
TEM
Transmission election microscopy → black and white image; vizualising cell organells
57
New cards
SEM
Scanning electron microscopy → black and white images; useful for scanning surfaces.
58
New cards
3D-imaging
Combination of histology and surface imaging: some examples
59
New cards
Name the 5 major classes of antibody molecule (immunoglobulins).
IgG, IgM, IgA, IgD and IgE and are classified according to the type of heavy chain constant region, and are distributed and function differently in the body.
60
New cards
What do these 5 immunoglobulins have in common?
\
\
They all have the same structures. They differ in amount of binding sites.
61
New cards
Why would one prefer an IgG antibody over an IgM antibody in immunohistochemistry?
IgG monoclonals are more popular because they are easier to generate and purify and then more stable to work with. They have higher affinity than IgM because they only have 2 binding sites. IgM has 10 binding sites which means that they replace low affinity with high avidity.
62
New cards
In which species are polyclonal antibodies in general raised?
\
\
Polyclonal antibodies have been raised in numerous species including, mice, rats, hamsters, guinea pigs, rabbits, goats, chickens, horses, donkeys, cattle, sheep and even emus. The use of the resulting antibody decides the choice of host.
63
New cards
Polyclonal antibody production
1\. Preparation of the antigen.
2\. Animal species selection.
3\. Preparation and selection of the adjuvant.
4\. Injection protocol.
5\. Post injection observation.
6\. Antibody collection.
64
New cards
You are involved in a study in which you would like to demonstrate that the granulosa cells of the porcine ovary express the protein anti-Mullerian hormone (AMH). AMH has been shown to be present in the rat ovary. The polyclonal AMH antibody (raised in rabbit) you have available is known to identify AMH in the rat, human and mouse ovary.
What steps would you undertake to determine whether this antibody can be used to identify AMH in the porcine ovary. Keep in mind that you indeed have to proof that the antibody also recognizes AMH in the porcine ovary (while answering this question also take into account the technique you learned in week 1 of the course).
* Antibody specificity can be assessed by comparing binding signals in cells expressing the target protein to control cells with the target gene knocked out by CRISPR or RNA interference (RNAi). A highly specific antibody should show no binding activity if the target isn't there.
* Also, a Western Blot can be performed to test AMH expression in a sample of cells. And if there is not a band at the kDa for AMH, but there is a target signal on those cells, then the antibody is not specific.
* Also, a Western Blot can be performed to test AMH expression in a sample of cells. And if there is not a band at the kDa for AMH, but there is a target signal on those cells, then the antibody is not specific.
65
New cards
You write a manuscript regarding AMH in the porcine ovary, but in contrast to the rat ovary you also find AMH immune staining in the thecal cells, surrounding the granulosa cells. The reviewer of your manuscript questions your conclusion that in the porcine ovary not only granulosa cells but also theca cells express AMH, he suggests that the AMH may have been secreted by the granulosa cells and taken up by the theca cells. What would you do to convince the reviewer that in the porcine ovary not only granulosa cells but also theca cells express AMH.
To determine AMH gene expression in theca (luteal) cells, first immunostaining was performed. After that Around 1x104 theca cells were collected and analysed separately from other kinds of cells. AMH gene expression in granulosa and theca cells, corpora lutea and liver (negative control) was analysed by qPCR. After that DNA gel electrophoresis was performed and visualised under UV light. So we should research AMH gene expression by qPCR in theca cells.
66
New cards
What are the major differences between monoclonal and polyclonal antibodies?
\
\
Monoclonal antibodies interact with the same epitope in the antigen whereas polyclonal antibodies interact with different epitopes of the same antigen.
67
New cards
What do we mean by Kohler illumination?
\
\
Illumination produced by a correctly positioned condenser lens that is uniformly bright and free from glare across the viewing area of the specimen. Different optical components are involved to control the illumination of the specimen
The collector/field lenses act to collect light from the source and focus it at the plane of the condenser diaphragm. The condenser lens acts to project this light, without focusing it, through the sample. The advantage is the uniform illumination of the sample meaning it provides high sample contrast.
The collector/field lenses act to collect light from the source and focus it at the plane of the condenser diaphragm. The condenser lens acts to project this light, without focusing it, through the sample. The advantage is the uniform illumination of the sample meaning it provides high sample contrast.
68
New cards
What is numerical aperature?
The numerical aperture (NA) of a microscopical lens (objective) is the measure of the ability of a lens to collect light from the specimen (section). Lenses with a low NA collect less light than the ones with a high NA. The higher the NA, the better the resolution of the lens will be. Will a low power lens have a high or low NA and thus a high or low resolution?
The numerical aperture (NA) is the measure of the ability of a lens to collect light from the specimen. The higher the numerical aperture of the total system, the better the resolution.
To answer the question: a lower power lens will have a lower NA and thus provide a lower resolution.
69
New cards
How to prevent double-labeling by fluorescent antibodies?
Use Cy5 (red) as it’s the farthest from FITC (turquoise), in relation to excitation and emission maximum.
70
New cards
For the H&E staining, should you use MilliQ water or tap water?
\
\
Tap water: contains minerals and salts which results in harsher staining of haemotoxylin because it has a low affinity ands needs salts and minerals to bind properly. Electrolytes will also make the staining darker. Tap water has chlorine which can bleach certain stains. pH is also fluctuating and can negatively affect the staining.
MilliQ water: does not contain minerals and salts and does not have a fluctuating pH.
MilliQ water: does not contain minerals and salts and does not have a fluctuating pH.
71
New cards
How to prevent auto-fluorescence?
Using different fluorophores or use a Sudan black dye.
72
New cards
Specimen Stains:
* Coloured dyes or stains can be used to introduce contrast into a specimen
* Different combinations of dyes may be used to stain various organelles in contrasting colours
* Bright-field imaging is typically used to observe these histological stains
* Immunofluorescence microscopy uses antibodies labelled with fluorescent probes
* Fluorescence in situ hybridization (FISH) is a technique that uses fluorescently labeled DNA or RNA probes
* Fluorescence analysis uses various fluorescent molecules such as antibodies, DNA or RNA probes
* Quantum dots are nanocrystals that emit different colors when exposed to laser light without photobleaching.
* Different combinations of dyes may be used to stain various organelles in contrasting colours
* Bright-field imaging is typically used to observe these histological stains
* Immunofluorescence microscopy uses antibodies labelled with fluorescent probes
* Fluorescence in situ hybridization (FISH) is a technique that uses fluorescently labeled DNA or RNA probes
* Fluorescence analysis uses various fluorescent molecules such as antibodies, DNA or RNA probes
* Quantum dots are nanocrystals that emit different colors when exposed to laser light without photobleaching.
73
New cards
What is fluorescence microscopy and how does it work?
Fluorescence microscopy is a widely used contrast technique due to its superior signal-to-noise ratios. It utilizes specific wavelengths of light to excite a fluorescent molecule or fluorophore in the specimen, and then images the longer wavelength light emitted from the excitation of the fluorophore. A set of filters are required to block all wavelengths of light except for the excitation wavelength, including an excitation filter, a dichromatic mirror, and a barrier filter. The fluorescence emitted from the specimen is detected by a sensitive digital camera.
74
New cards
What is differential interference contrast (DIC) and when is it used?
Differential interference contrast (DIC) is a form of interference microscopy that produces images with a shadow relief. It is used for viewing unstained cells in tissue culture, eggs and embryos, and in combination with some stains. It allows the overall shape and relief of the structure to be viewed and can be used with a subset of the structure stained with a coloured dye.
75
New cards
What is phase contrast and when is it used?
Phase contrast is a method used for imaging unstained cells in tissue culture and for testing cell and organelle preparations for lysis. It uses differences in the refractive index of cellular structures to produce contrast. A specialised phase condenser and phase objective lenses are required, and each phase setting of the condenser lens is matched with the phase setting of the objective lens.
76
New cards
What is dark-field illumination and when is it used?
Dark-field illumination is a configuration of the light microscope that produces images of brightly illuminated objects on a black background. It is traditionally used for viewing the outlines of objects in liquid media such as living spermatozoa, microorganisms, cells growing in tissue culture, or for a quick check of the status of a biochemical preparation.
77
New cards
What are the different types of optical contrast in microscopy?
The different types of optical contrast in microscopy are:
1. Bright-field illumination
2. Dark-field illumination
3. Phase contrast
4. Differential interference contrast (DIC)
5. Fluorescence microscopy
1. Bright-field illumination
2. Dark-field illumination
3. Phase contrast
4. Differential interference contrast (DIC)
5. Fluorescence microscopy
78
New cards
Why is it necessary to introduce contrast into a specimen when viewing it in a light microscope?
Most cells and tissues lack contrast when viewed in a light microscope because they are colorless and almost transparent. Therefore, it is necessary to introduce contrast into the specimen either by optical means or by staining the specimen with a dye, or a combination of both.
79
New cards
In order to collect images from the specimen/sample, what is necessary?
* The specimen must be in a form that is compatible with the microscope.
* \
* \
80
New cards
What is the goal of a specimen preparation protocol?
* The goal is to render the tissue of interest into a form for optimal study with the microscope.
\
\
81
New cards
What is the purpose of a coverslip?
\
\
* The coverslip protects the specimen and helps achieve optimal resolution.
\
\
82
New cards
What are coverslips graded by?
\
\
* Coverslips are graded by their thickness, with the thinnest ones labelled #1.
* \
* \
83
New cards
What is essential when using a coverslip with a high-magnification objective lens?
\
\
* It is essential to use a coverslip that is matched to the objective lens.
\
\
84
New cards
Why is it necessary to seal the coverslip to the glass slide?
\
\
* Shear forces from the movement of the coverslip over the glass slide can cause damage to the specimen or the objective lens.
\
\
85
New cards
How are cells kept alive on the stage of the microscope?
\
\
* They are usually mounted in some form of chamber, and if necessary, heated.
\
\
86
New cards
What is a microtome used for?
\
\
* A microtome is used to cut specimens into thin sections for imaging.
\
\
87
New cards
What are fixatives used for in specimen preparation?
\
\
* Fixatives are used to preserve the structural integrity of the tissue.
\
\
88
New cards
What is permeabilization in specimen preparation?
\
\
* Permeabilization is the process of allowing stains to infiltrate the tissue after fixation.
\
\
89
New cards
What are some preparation protocols developed to improve the visibility of biological structures in specimens?
CLARITY and expansion microscopy are preparation protocols developed to improve the visibility of biological structures in specimens.
90
New cards
Digital microscopy
\
Definition: The use of digital cameras and software to capture, store, and analyze microscope images. Digitally captured images can be displayed on a computer screen, stored in a digital format, and reproduced using digital methods.
Definition: The use of digital cameras and software to capture, store, and analyze microscope images. Digitally captured images can be displayed on a computer screen, stored in a digital format, and reproduced using digital methods.
91
New cards
Latex labelling
A method of linking antibodies to latex particles for use in immunoassays or lateral flow devices
92
New cards
Limit of resolution
\
\
The smallest distance that two objects can be separated and still be distinguished as separate objects in the microscope image. The limit of resolution for a microscope that uses visible light is about 300 nm with a dry lens and 200 nm with an oil immersion lens.
93
New cards
Rare earth metals
Elements used as labels and attached to antibodies as a chelate for multiplexing in a single assay
94
New cards
Gold labelling
A method of linking antibodies to gold particles for use in immunosorbent electron microscopy and lateral flow devices
95
New cards
Immunoassays Definition:
\
\
Techniques used to detect and quantify antigens using antibodies
96
New cards
Direct labelling
Method of attaching labels to primary antibodies via a covalent bond
97
New cards
Indirect labelling
Method of attaching labels to secondary antibodies that bind to the primary antibody-antigen complex
98
New cards
Marker enzymes
Enzymes like horseradish peroxidase or alkaline phosphatase that can be linked to antibodies for detection
99
New cards
Fluorescent labels
Labels that emit light when excited with a specific wavelength of light, commonly used in immunofluorescence assays
100
New cards
Biotin-streptavidin interaction
A strong non-covalent bond used to link biotinylated antibodies to streptavidin-conjugated enzymes or fluorophores