IB Chemistry 2025 Curriculum Guide
1/429
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
430 Terms
Element
Basic substance that cannot be chemically broken down.
Compound
Chemically bonded atoms of different elements.
Mixture
Combination of elements or compounds without fixed ratios.
Homogeneous Mixture
Uniform composition throughout the mixture.
Heterogeneous Mixture
Non-uniform composition with distinguishable components.
Solvation
The process of surrounding solute particles with solvent particles to form a solution
filtration
A process that separates materials based on the size of their particles.
Recrystallization
The process by which bonds between atoms in minerals break and re-form in new ways during metamorphism.
distillation
A process that separates the substances in a solution based on their boiling points
paper chromatography
laboratory technique used to observe the different pigments in a material
what factors are considered in choosing a method to separate the components of a mixture?
the type of mixture, the physical properties of the components, and the desired outcome
How do intermolecular forces influence the type of mixture that forms between two substances?
Intermolecular forces determine whether a solution is homogeneous or heterogeneous. The strength and type of intermolecular forces between the molecules of the solute and solvent determine their ability to mix and form a stable solution
Why are alloys generally considered to be mixtures, even though they often contain metallic bonding?
Because the constituent elements are physically mixed rather than chemically combined in a compound. They retain the individual properties of the original metals, unlike compounds which form a new substance with distinct properties
Kinetic Molecular Theory
Model explaining properties of matter (solids, liquids, gases) and changes of state
States that the theory that all matter is composed of particles (atoms and molecules) moving constantly in random directions
Melting
solid to liquid
Freezing
liquid to solid
vaporization/evaporation/boiling
Liquid to gas
condensation
Gas to liquid
Sublimination
solid to gas
Deposition
gas to solid
Why are some substances solid while others are fluid under standard conditions?
Because of a balance between the kinetic energy of their particles and the strength of intermolecular forces. Solids have strong intermolecular forces, keeping particles closely packed and in fixed positions. Liquids have weaker forces, allowing particles to move past each other. Gases have very weak forces, with particles moving independently and widely spaced.
Why are some changes of state endothermic and some exothermic?
- endothermic (absorb heat): involve breaking bonds to transition from a more ordered state to a less ordered state, like melting or boiling.
- exothermic (release heat): involve forming bonds to transition from a less ordered state to a more ordered state, like freezing or condensation
Endothermic Change
Process absorbing heat during a state change.
Exothermic Change
Process releasing heat during a state change.
Temperature
Measure of average kinetic energy of particles.
Observable changes in physical properties and temperature during changes of state.
Shape, volume, and temperature will change as the substance transitions between solid, liquid, and gas states
What is the graphical distribution of kinetic energy values of particles in a sample at a fixed temperature?
Maxwell-Boltzmann distribution. It shows how many particles have a certain kinetic energy. The shape of the distribution is determined by the temperature
What must happen to particles for a chemical reaction to occur?
particles must collide with sufficient energy and the correct orientation ("effective" collisions). They lead to the breaking of old bonds and the formation of new ones
Nuclear Atom
Atom with a positively charged, dense nucleus composed of protons and
neutrons (nucleons). Negatively charged electrons occupy the space outside the nucleus.
nuclear symbol
- the superscript (A): the mass number (= protons + neutrons)
- the subscript: the atomic number (= protons)
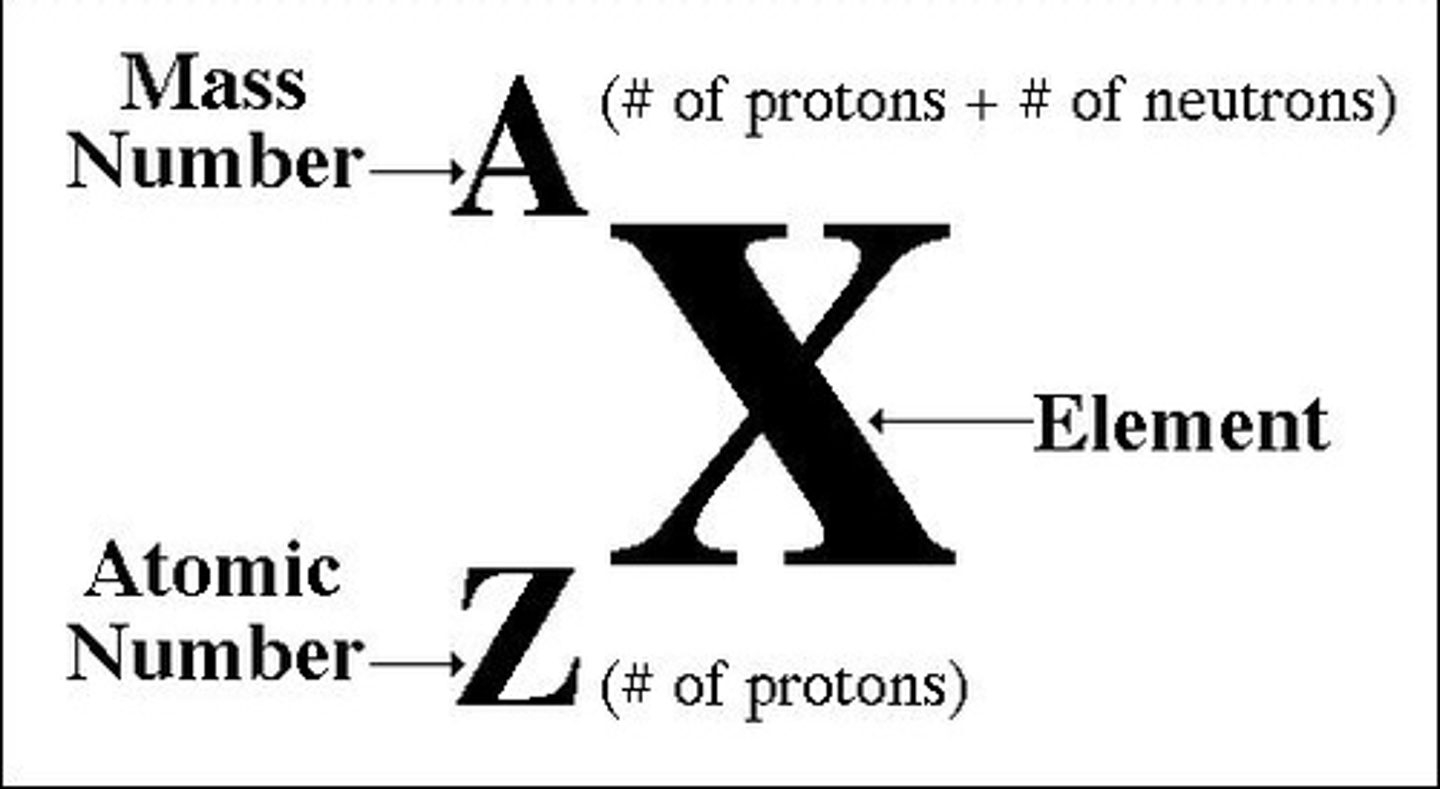
What determines the different chemical properties of atoms?
Its valence electrons (the electrons in the outermost shell). The number and arrangement of these electrons dictate how an atom interacts with other atoms, influencing its reactivity and bonding behavior
How does the atomic number relate to the position of an element in the periodic table?
Elements are arranged in order of increasing atomic number. This arrangement is fundamental to the structure of the periodic table, as it also reflects the arrangement of electrons in the atom
physical properties
A characteristic of a pure substance that can be observed without changing it into another substance
-ex: color, density, hardness, and melting and boiling points
chemical properties
Characteristic that cannot be observed without altering the substance. They will determine how it will react with other substances.
-ex: flammability, reactivity, solubility, heat from combustion, types of chemical bonds formed, oxidization states, and acidity/basicity
Isotope
Atoms of the same element with different numbers of neutrons.
Isotope abundance
percentage of that isotope that occurs naturally in an element
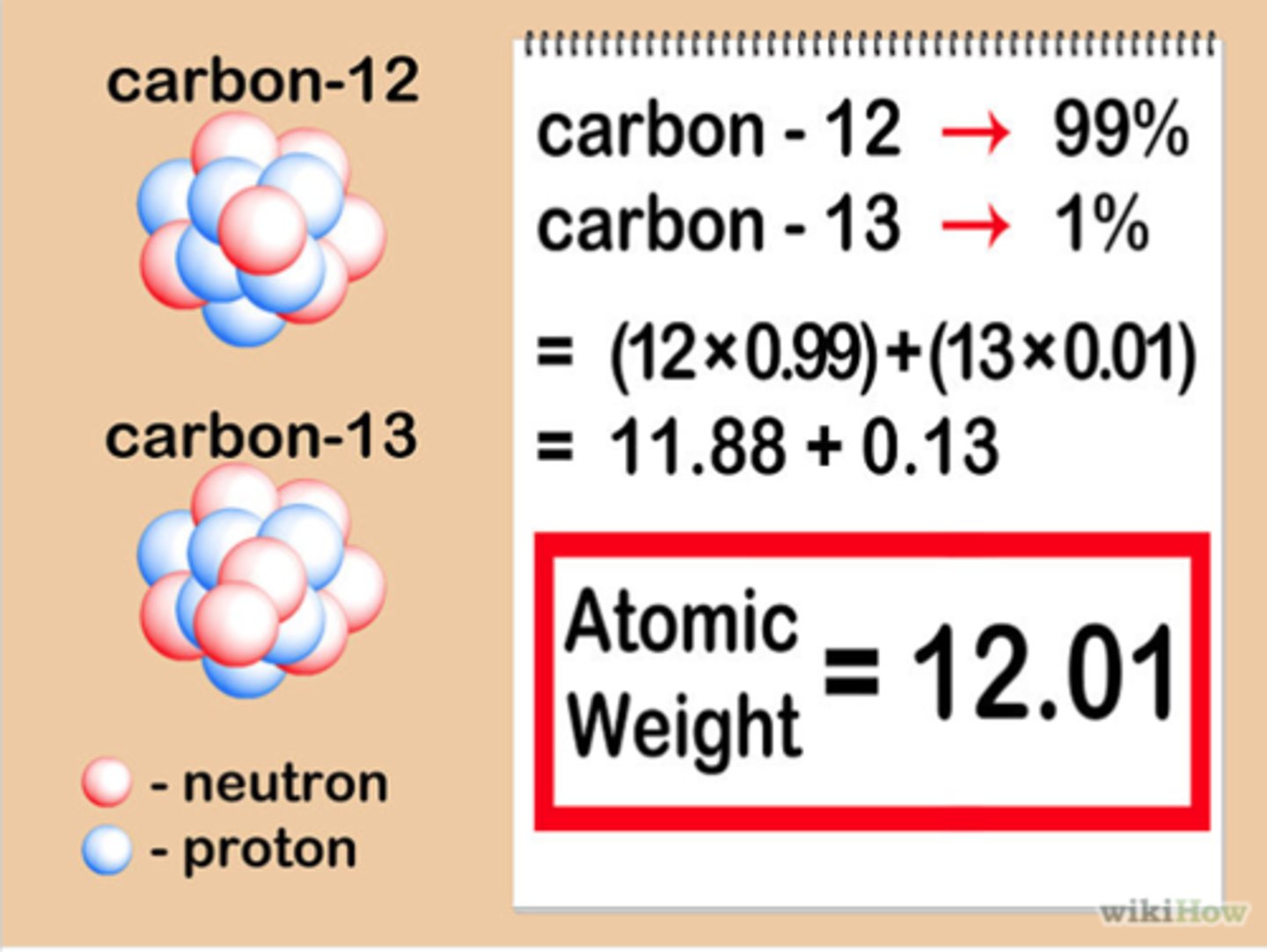
Differences in the physical properties of isotopes
Isotopes of the same element have the same chemical properties but different physical properties because they differ in mass, which affects their density, boiling point, and melting point. The physical properties of an isotope are largely determined by its atomic mass, which is directly related to the number of neutrons in its nucleus
How can isotope tracers provide evidence for a reaction mechanism?
Isotope tracers (atoms of a substance labeled with a different isotope) track the movement of atoms/molecules during a reaction. This is achieved by observing how the tracer isotope behaves differently compared to the normal isotope.
Electron Configuration
Distribution of electrons in an atom's energy levels.
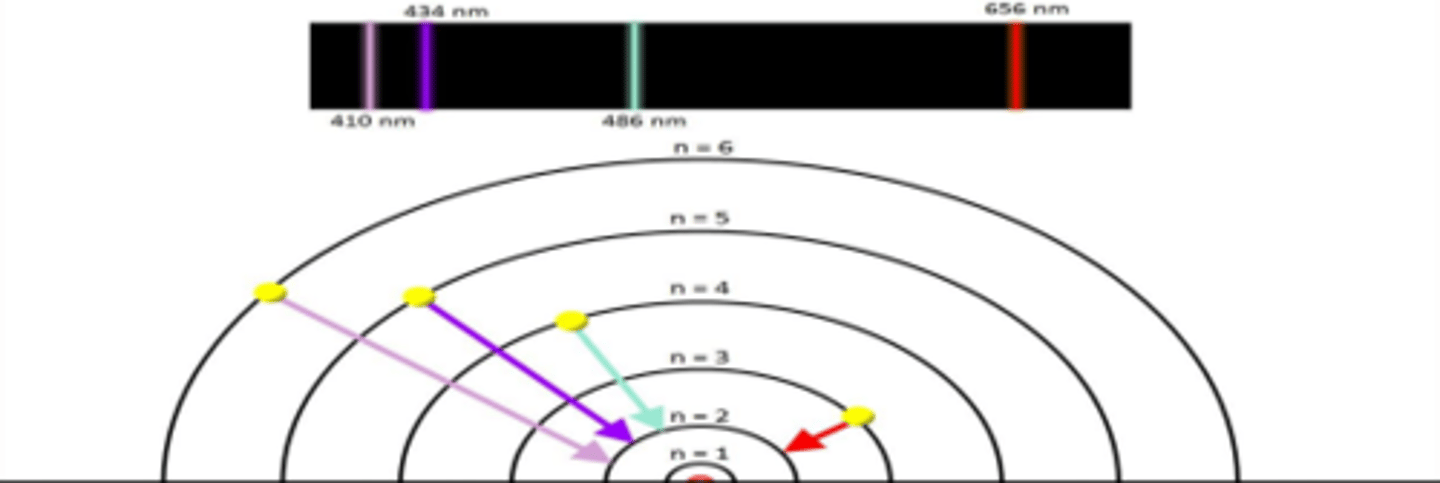
Emission Spectrum
Produced by atoms emitting photons when electrons in excited states return to lower energy levels.
Photon
A particle representing a quantum of light.
Continuous Spectrum
Shows all wavelengths without gaps.
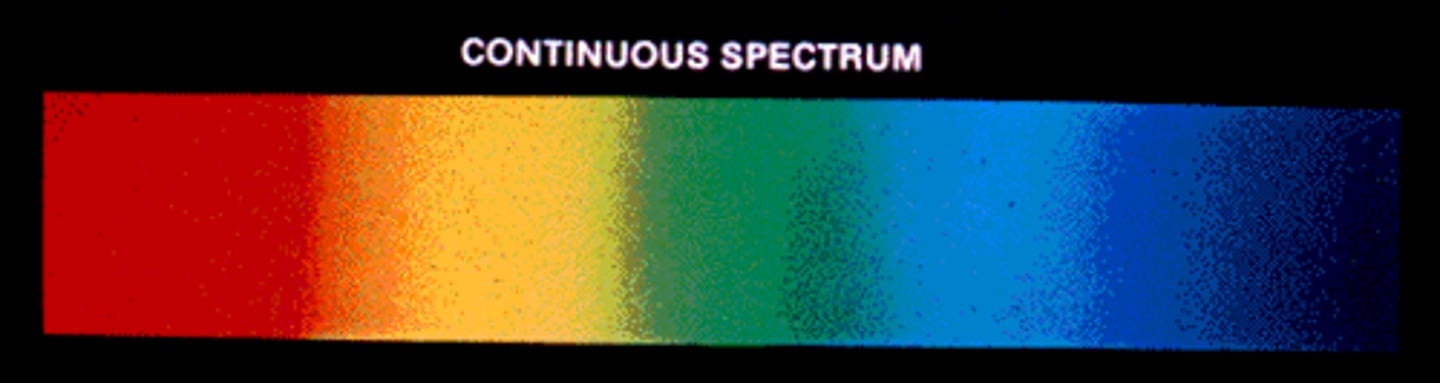
Line Spectrum
Displays discrete energy levels, which converge at higher energies
Discrete Energy Levels
Specific energy states electrons can occupy.
wavelength vs frequency/energy
inversely proportional
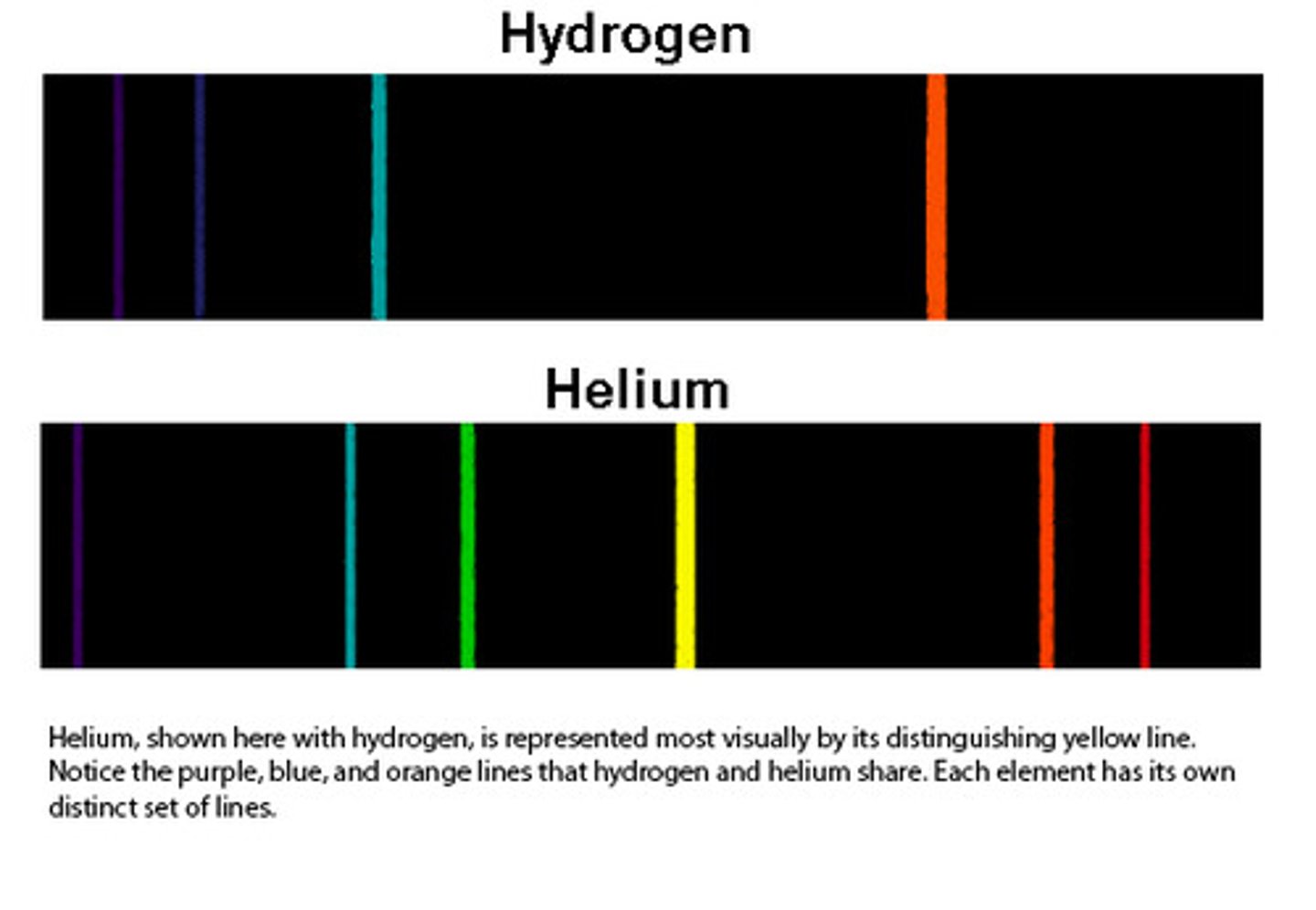
Hydrogen Emission Spectrum
Evidence of electrons in quantized energy levels.
In the study of emission spectra from gaseous elements and of light, what qualitative and quantitative data can be collected from instruments such as gas discharge tubes and prisms?
- Qualitatively, they reveal the unique line spectrum patterns of different elements, allowing for element identification.
- Quantitatively, they can be used to measure the intensity and wavelength of emitted light, potentially indicating the concentration of an element or its energy level
How do emission spectra provide evidence for the existence of different elements?
Each element emits light at specific wavelengths when its electrons transition between energy levels. These unique patterns of emitted light (line spectra) allow scientists to identify and differentiate between elements. By analyzing the wavelengths of light emitted, scientists can determine which elements are present in a sample
Main Energy Level/Maximum # of elections
Integer number n representing electron shells.
- Maximum = 2n² for each energy level.
How does an element's highest main energy level relate to its period number in the periodic table?
An element's period number in the periodic table directly corresponds to the highest main energy level (or shell) occupied by its electrons. In simpler terms, the period number tells you which electron shell is the outermost and contains the valence electrons. For example, an element in period 3 has its outermost electrons in the third energy level
Sublevels
Divisions of main energy levels into s, p, d, f.
s Orbital
Spherical shape, holds up to 2 electrons.
p Orbitals
Three orientations, each holds 2 electrons.
What is the relationship between energy sublevels and the block nature of the periodic table?
The periodic table's block structure (s, p, d, and f blocks) directly reflects the way electrons fill energy sublevels in atoms. Each block corresponds to a specific type of sublevel being filled at that point in the periodic table.
Aufbau Principle
Electrons fill lower energy orbitals first.
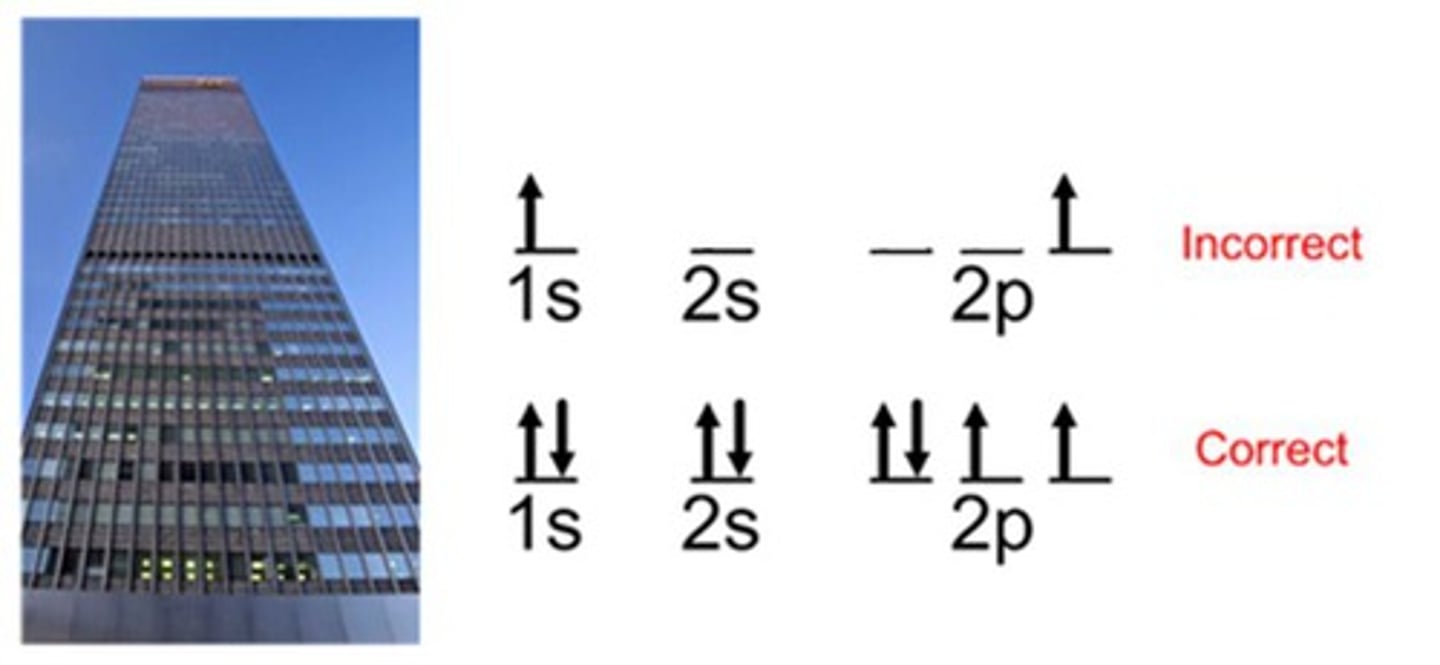
Hund's Rule
orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin
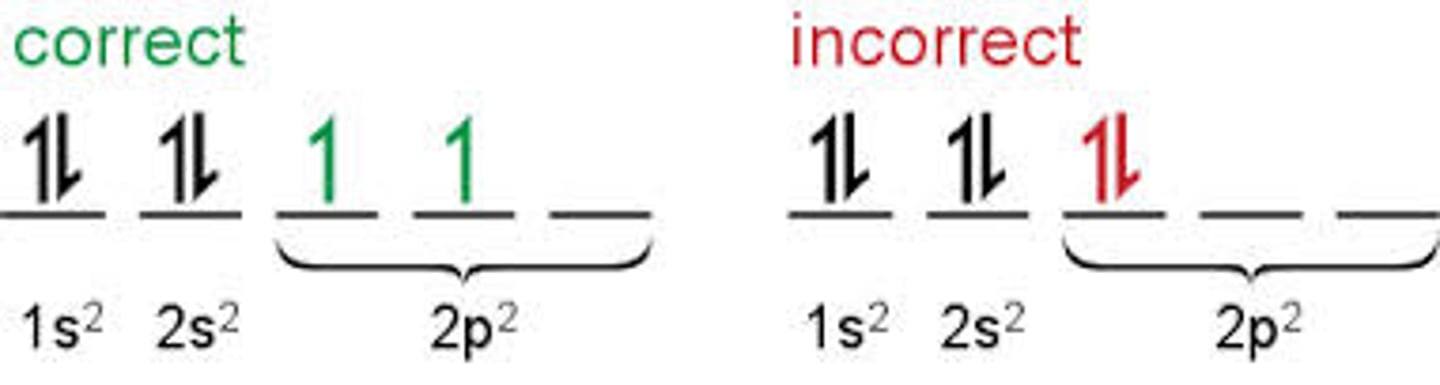
Pauli Exclusion Principle
An atomic orbital may describe at most two electrons, each with opposite spin direction
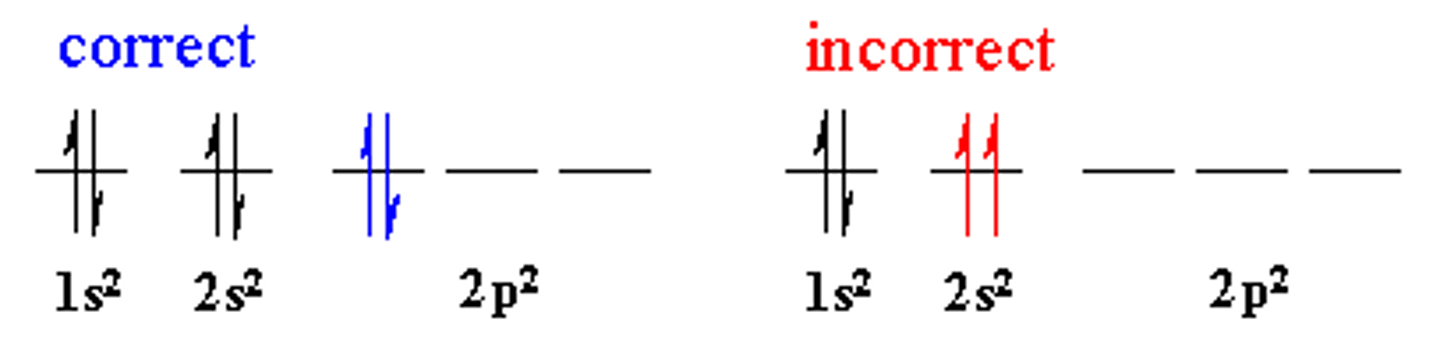
Orbital Diagram
Visual representation of electron configurations in orbitals.
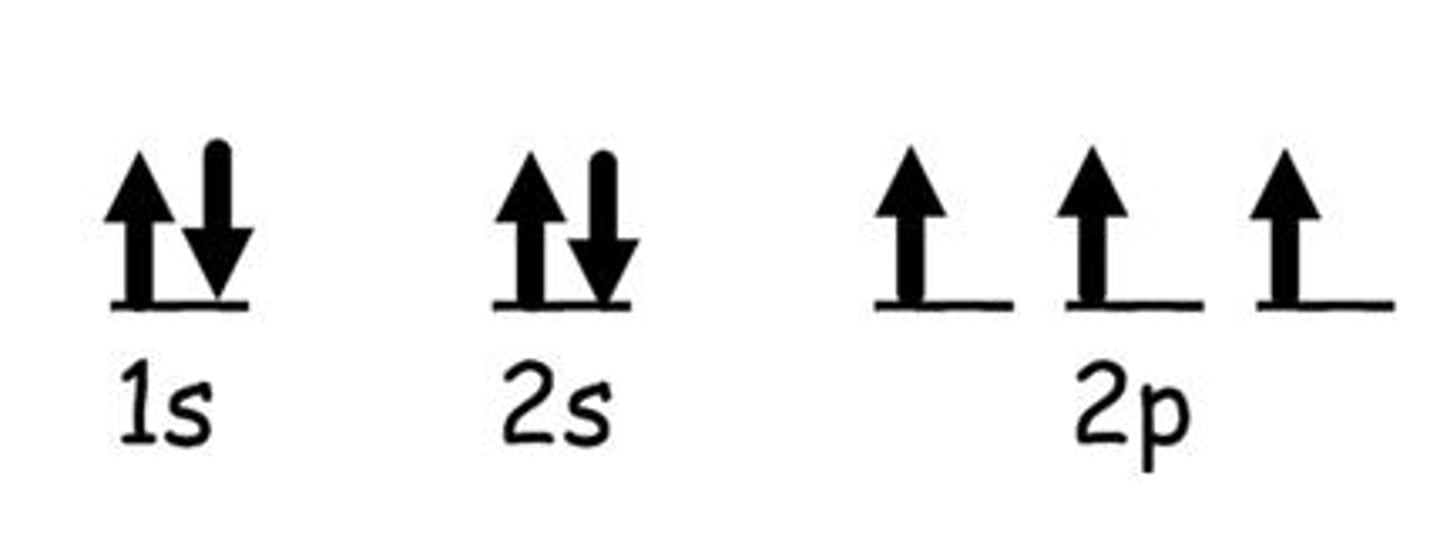
electron configurations exceptions
- Cr: [Ar] 3d5 4s1
- Cu: [Ar] 3d10 4s1
WHY: Transition metal exceptions occur because half-filled or fully filled subshells (like d orbitals) exhibit greater stability than partially filled ones.
![<p>- Cr: [Ar] 3d5 4s1</p><p>- Cu: [Ar] 3d10 4s1</p><p>WHY: Transition metal exceptions occur because half-filled or fully filled subshells (like d orbitals) exhibit greater stability than partially filled ones.</p>](https://knowt-user-attachments.s3.amazonaws.com/e3889bec-75b8-4ced-a1d0-2aafa14eaae6.jpg)
Avogadro Constant (NA)
Number of entities in one mole, 6.022 x 10²³.
Elementary Entity
An atom, molecule, ion, or particle
Relative Atomic Mass (Ar)
The weighted mean mass of an atom of an element compared with one-twelfth of the mass of an atom of carbon-12.
Atoms increase in mass as their position descends in the periodic table. What properties might be related to this trend?
Atomic Radius: increases as you move down a group due to the addition of more electron shells, which push the valence electrons further from the nucleus.
Ionic Radius: increases as you move down a group due to the addition of electron shells.
Ionization Energy: (the energy required to remove an electron) decreases as you move down a group. This is because the valence electrons are further from the nucleus and experience less attraction.
Electron Affinity: (the energy change when an atom gains an electron) decreases as you move down a group. The larger atomic size makes it easier to add an electron, but the change in electron affinity is less pronounced than ionization energy.
Electronegativity: (the ability of an atom to attract electrons in a chemical bond) decreases as you move down a group. This is because the valence electrons are further from the nucleus and have less attraction.
Relative Formula Mass (Mr)
the total of the relative atomic masses, added up in the ratio shown in the chemical formula, of a substance
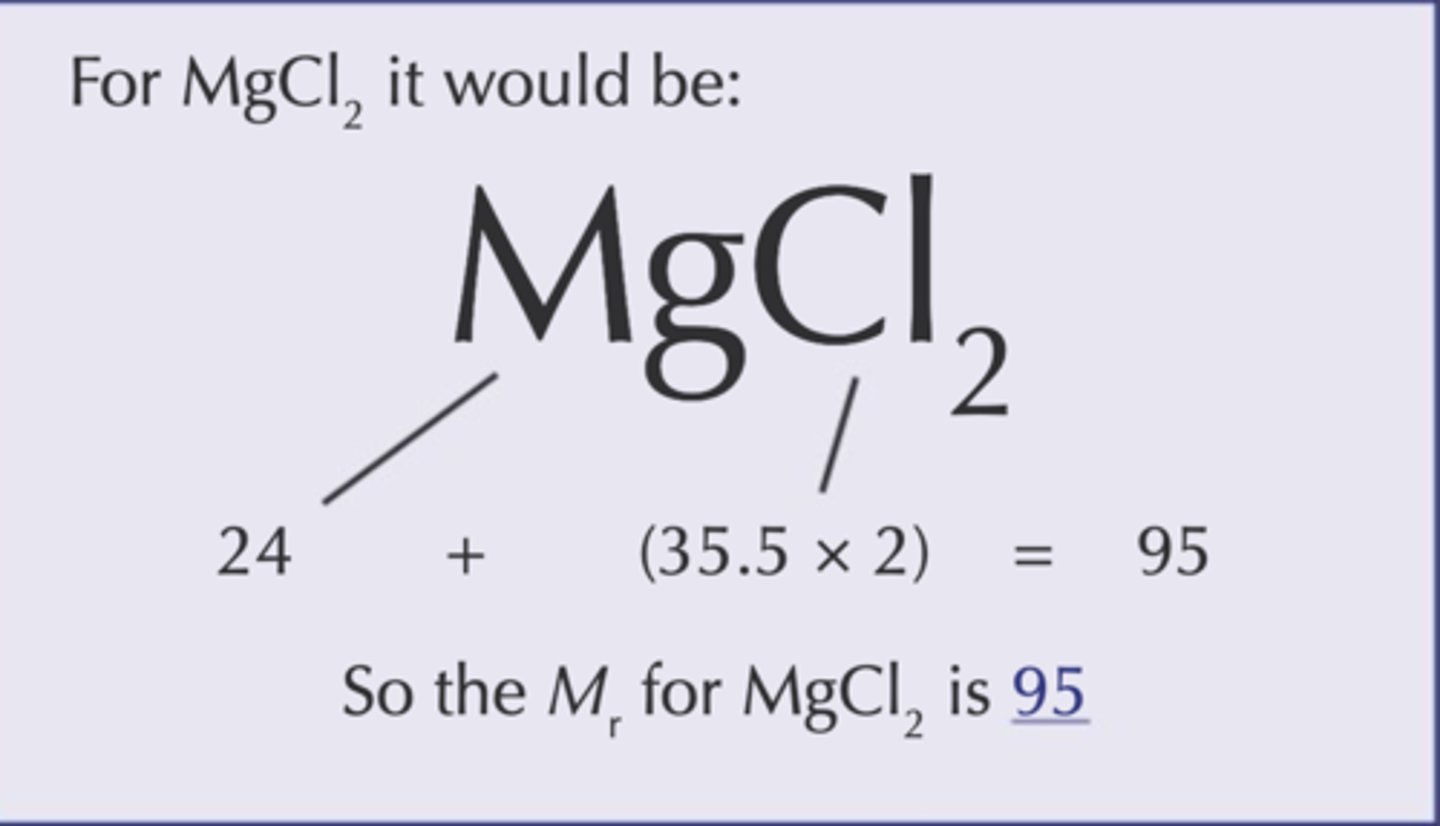
Molar Mass (M)
The mass mole of a substance
- units: g/mol
How can molar masses be used with chemical equations to determine the masses of the products of a reaction?
(with balanced chemical equations) are used to determine the masses of products formed in a reaction through stoichiometry. This involves converting given masses of reactants into moles, using the balanced equation's mole ratios, and then converting the resulting moles of products back into mass.
percentage composition
the percentage by mass of each element in a compound
How can experimental data on mass changes in combustion reactions be used to derive empirical formulas?
by analyzing the masses of combustion products (like CO2 and H2O) to determine the masses and then moles of the original elements (like C and H). This process involves calculating the number of moles of each element, finding their ratios, and then expressing those ratios as a whole number ratio to form the empirical formula
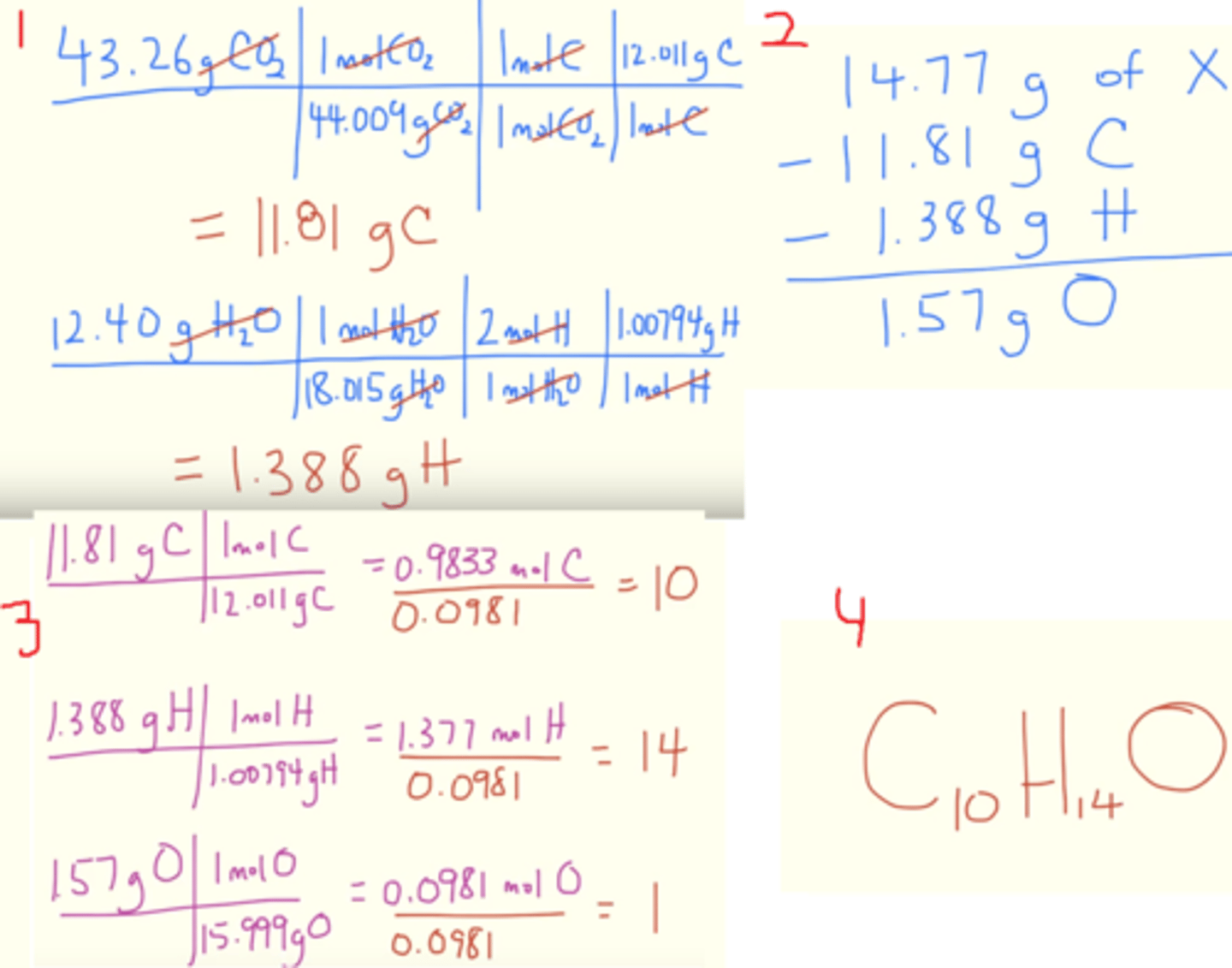
What is the importance of approximation in the determination of an empirical formula?
because experimental measurements often have small errors. It allows slight deviations from whole numbers to be rounded appropriately to find the simplest whole-number ratio of atoms. Without approximation, minor inaccuracies could lead to incorrect or overly complicated formulas.
molar concentration (molarity)
the amount of solute per volume of solution
- units: mol/dm^3 (or mol/L) and g/dm^3
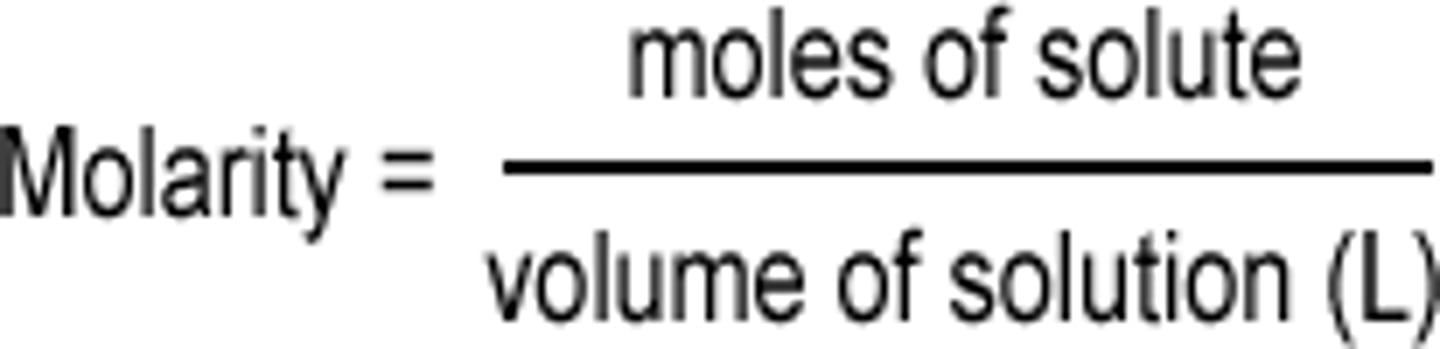
dm^3 to L
1 dm^3 = 1 L
dm^3 to cm^3
1 dm^3 = 1000 cm^3
1 cm^3 to ml
1 cm^3 to 1 mL
n = CV
moles = concentration x volume
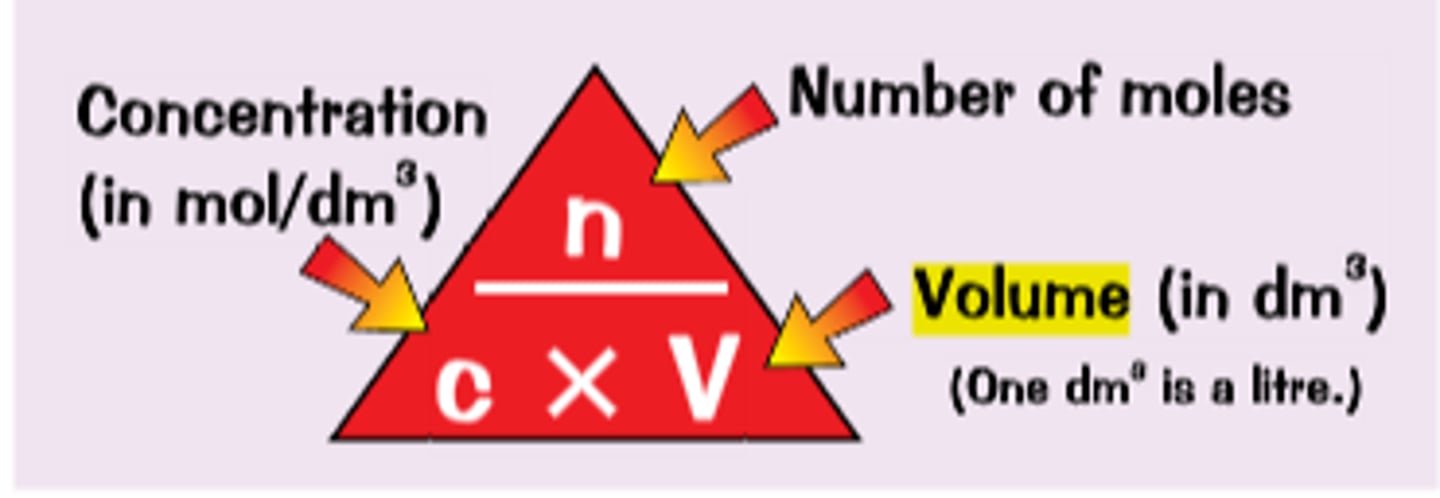
What are the considerations in the choice of glassware used in preparing a standard solution and a serial dilution?
When choosing glassware for preparing standard solutions and serial dilutions, accuracy, precision, and minimizing error are key considerations. Volumetric flasks, known for their precise volume measurements, are preferred for standard solution preparation. For serial dilutions, where multiple dilutions are needed, smaller, more manageable volumes are often used.
How can a calibration curve be used to determine the concentration of a solution?
used to determine the concentration of a solution by comparing the measured response of a sample to a graph of known concentrations. This graph, also known as a standard curve, plots the measured response (like absorbance or electrical signal) against the corresponding concentration of a series of known standard solutions. The concentration of an unknown sample can then be determined by interpolating its measured response on the calibration curve.
Avogadro's Law
equal volumes of all gases measured under the same
conditions of temperature and pressure contain equal numbers of molecules
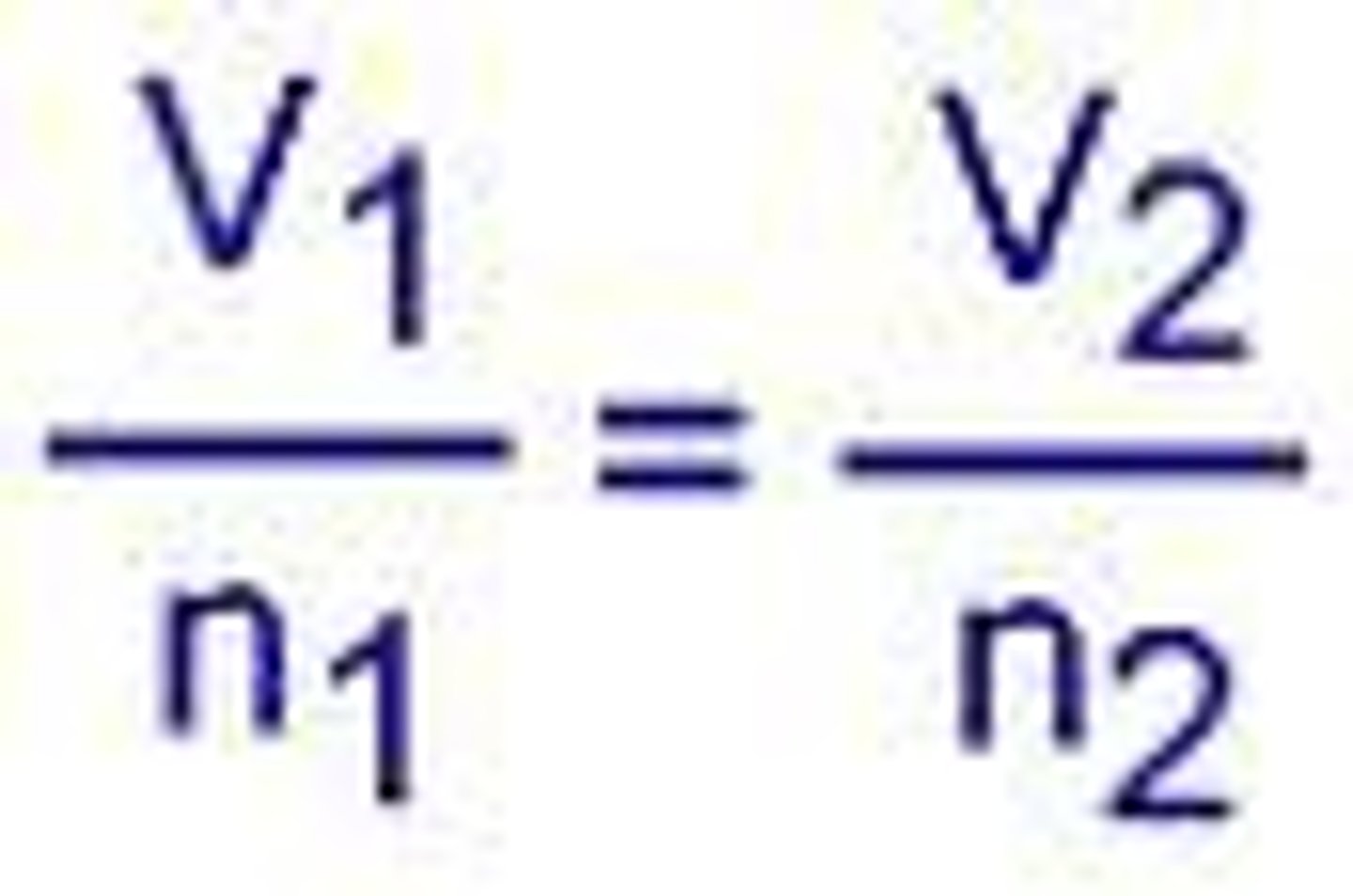
Avogadro's law applies to ideal gases. Under what conditions might the behavior of a real gas deviate most from an ideal gas?
Real gases deviate most from ideal gas behavior at low temperatures and high pressures. These conditions intensify the influence of intermolecular forces and the finite volume of gas molecules, which are not accounted for in the ideal gas law.
Ideal Gas
Has moving particles with negligible volume and no intermolecular forces. All collisions between particles are considered elastic (collisions where total kinetic energy is conserved)
Real Gas
Gas that deviates from ideal behavior under certain conditions, particularly at low temperature and high pressure.
Ideal Gas Law Limitations
It accurately describes gas behavior at low pressures and high temperatures, but fails to account for real gas characteristics like molecular volume and intermolecular forces, which become significant at high pressures and low temperatures.
Under comparable conditions, why do some gases deviate more from ideal behavior than others?
because of the strength of their intermolecular forces and the size of their molecules. Gases with stronger intermolecular forces and larger molecules tend to deviate more from ideal gas behavior, especially at low temperatures and high pressures.
Molar Volume of an ideal gas
the volume occupied by 1 mole of a gas at standard temperature and pressure (STP); 22.7 dm^3/mol
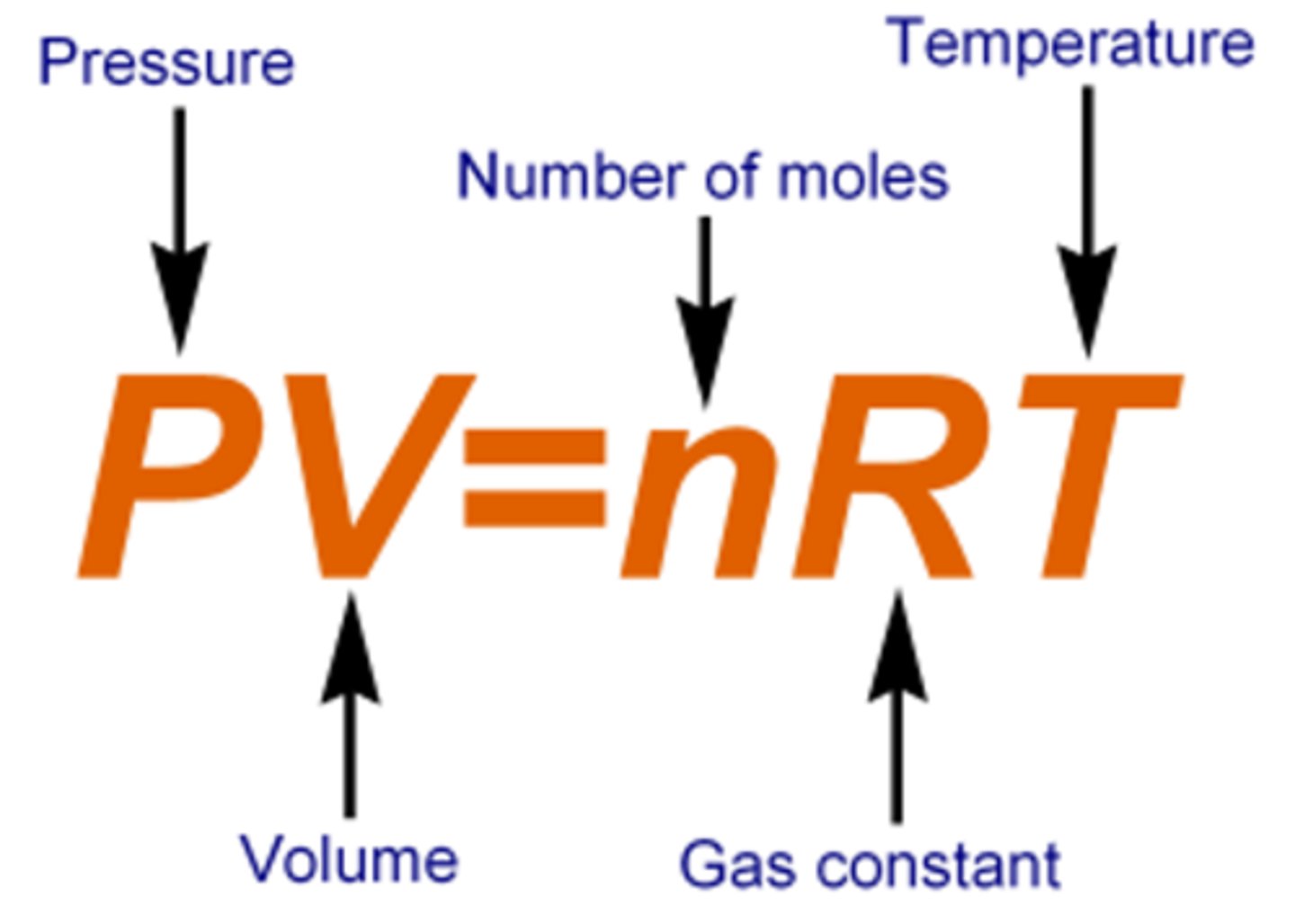
Ideal Gas Equation
PV = nRT relates pressure, volume, temperature.
Combined Gas Law
P1V1/T1 = P2V2/T2 for gas conditions.
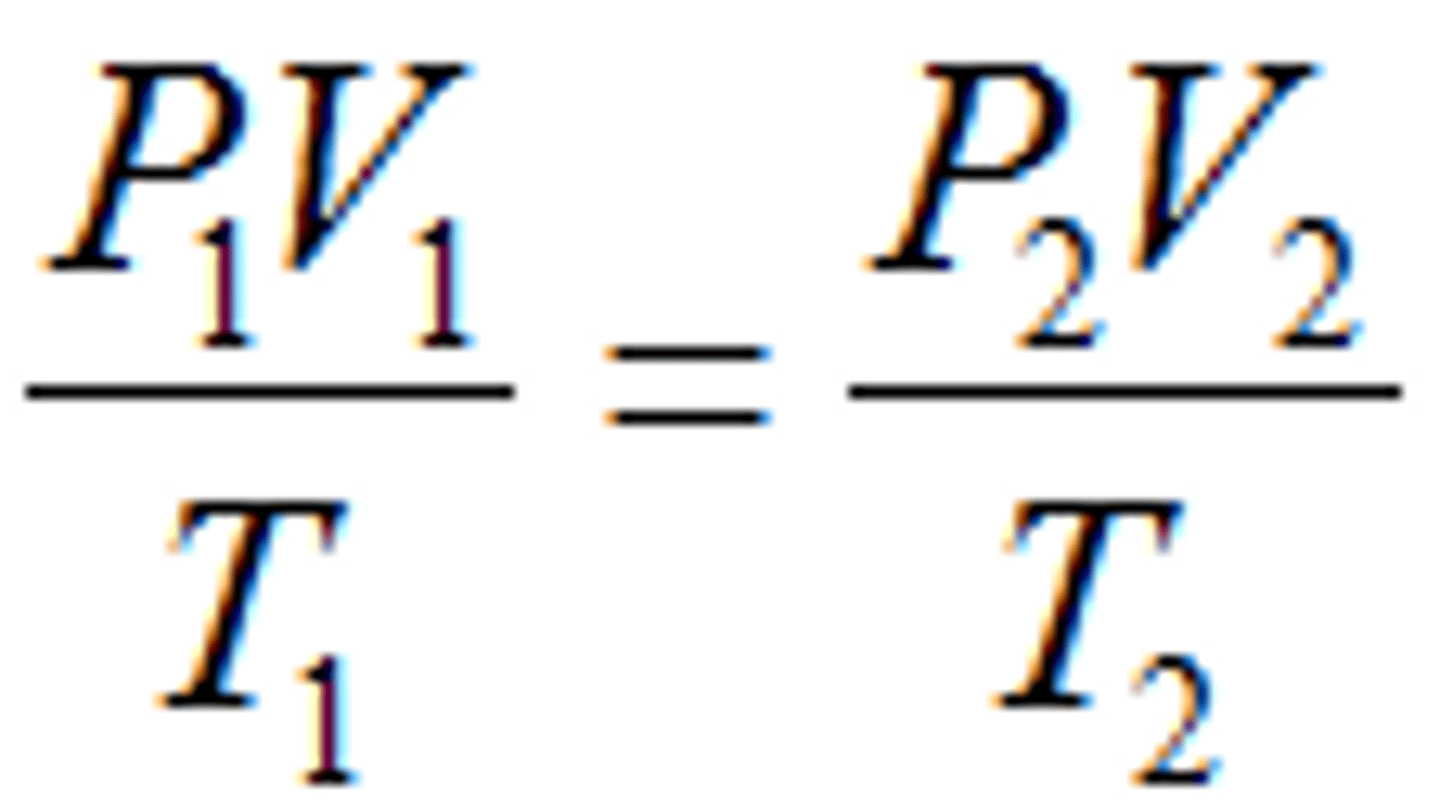
Mole Ratio
Ratio of moles of reactants/products in reactions.
Solute
Substance dissolved in a solution.
Cation
Positive ion formed by losing electrons.
Anion
Negative ion formed by gaining electrons.
How does the position of an element in the periodic table relate to the charge of its ion(s)?
An element's position on the periodic table helps predict the charge of its ions, primarily due to the element's tendency to gain or lose electrons to achieve a stable electronic configuration, similar to a noble gas. Metals generally lose electrons to form cations, while nonmetals tend to gain electrons to form anions. The charge of an ion is often related to the element's group number on the periodic table.
Ionic Bond
Electrostatic attraction between oppositely charged ions.
Binary Ionic Compounds
Composed of two different elements
- naming: cation first, followed by the anion with the suffix "ide"
Polyatomic Ions
• ammonium NH4 +
• hydroxide OH -
• nitrate NO3 -
• hydrogencarbonate HCO3 -
•carbonate CO3 2-
• sulfate SO4 2-
• phosphate PO4 3-
Why is the formation of an ionic compound from its elements a redox reaction?
because it involves the transfer of electrons between the reacting elements. During the formation of an ionic compound, one element loses electrons (oxidation) and another element gains those electrons (reduction).
physical properties of ionic compounds
- Volatility: low volatility
- Solubility: in ionic or polar solvents but NOT in non polar solvents
- Electrical Conductivity: when in liquid state (molten or aqueous solution)
- MP/BP: Very high - electrostatic attraction between ions in lattice are strong
Lattice Structure
Three-dimensional arrangement of ions in ionic compounds (represented by empirical formulas)
Lattice Enthalpy
Measure of ionic bond strength, influenced by ion radius and charges.
How can lattice enthalpies and the
bonding continuum explain the trend in melting
points of metal chlorides across period 3?
melting points of metal chlorides across Period 3 show a general increase from sodium chloride to magnesium chloride, and then a decrease to aluminum chloride, due to the interplay of lattice enthalpy and covalent character in the bonding
Covalent bond
formed by the electrostatic attraction between a shared pair of electrons and the positively charged nuclei
Octet Rule
Atoms tend to achieve eight electrons in valence shell.