Neuroanatomy 3 -- development of nervous system 2 Histogenesis in the neural tube –
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Histogenesis steps in the neural tube –
1. Interkinetic nuclear migration
2. Symmetric vs asymmetric division
3. Formation of 3 layers
4. Differentiation of nerve cells
5. Myelination
Neural tube histology
From a simple columnar epithelium to a mitotically active pseudostratified epithelium (neuroepithelium)
Methods for cell number increase in the neuroepithelium
Interkinetic nuclear migration in the neuroepithelium –
Consisting of a Pial/basal and a ventricular/apical side
Nucleus moving between sides w/ cell cycle
Process in which nucleus migrates in the cytoplasm of elongated neuroepithelial progenitor cells (in phase with mitosis)
Thus pseudostratified — nuclei @ different heights & mitotically active
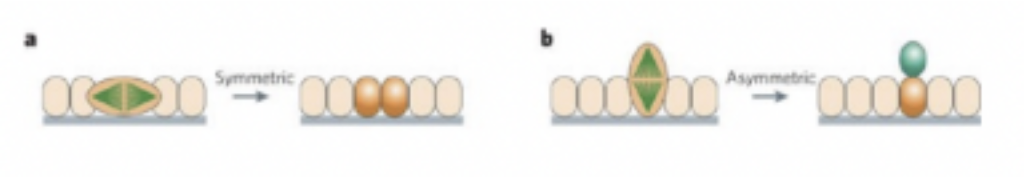
Symmetric cell division –
Generates two morphologically similar daughter cells that are both likely to be stem cells, thus increasing the precursor cell population — creates radial glial cells
Evolution of primary neural precursor cells into ventricular radial glial cells
Called this because these projections of the cytoplasm seems like rays in the tube (originally thought to be glial cells but then discovered to be precursor cells) – simple precursor cells that become radial glial cells that — (at this point due to symmetric cell division, but now) —
Ventricular radial glial cells undergo an asymmetric mitotic division — generate a progenitor cell and a differentiating cell
Thus increasing cell #
In symmetric division — mitotic spindle (pulling them apart) parallel to axis of epithelium — in asymmetric — perpendicular
Differentiating cells migrate to their final position using radial glia (wraps around cytoplasmic projections of radial glia cells and uses them to migrate to its final position)
Defects in migration can cause lissencephaly — ex. remain where they are & thus cause much bigger ventricles
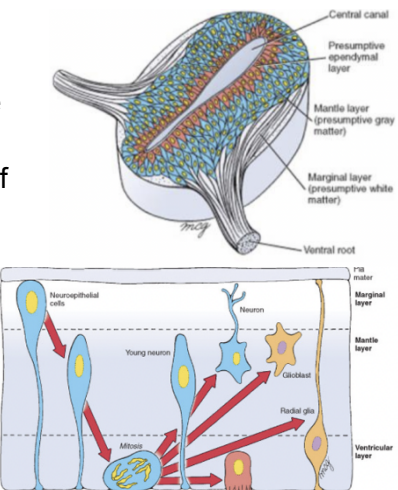
Generation of neurons and glial cells and organized in the wall of the neural tube into 3 zones –
Ventricular/Ependymal layer —
Innermost, lined by ependymal cells
Mantle layer
Presumptive gray matter — most of the body’s neurons
Marginal layer
Presumptive white matter — made of axons
This organization is evident in the beginning, but over time its remnants are only visible in the spinal cord
Differentiation of nerve cells and neural circuits is a long process (neurons) –
At the beginning called neuroblasts, but then differentiate into much more complex structure, growing dendrites in all different directions, then into full fledged neuron
Takes place in many phases – both prenatally and postnatally
Production of neurons & glia, cell migration, and onset of molecular differentiation & connectivity occur prenatally
Unique cellular patterns & neurochemical maturation — around time of birth
Extensive neuronal growth, synaptic formation & refinement postnatally
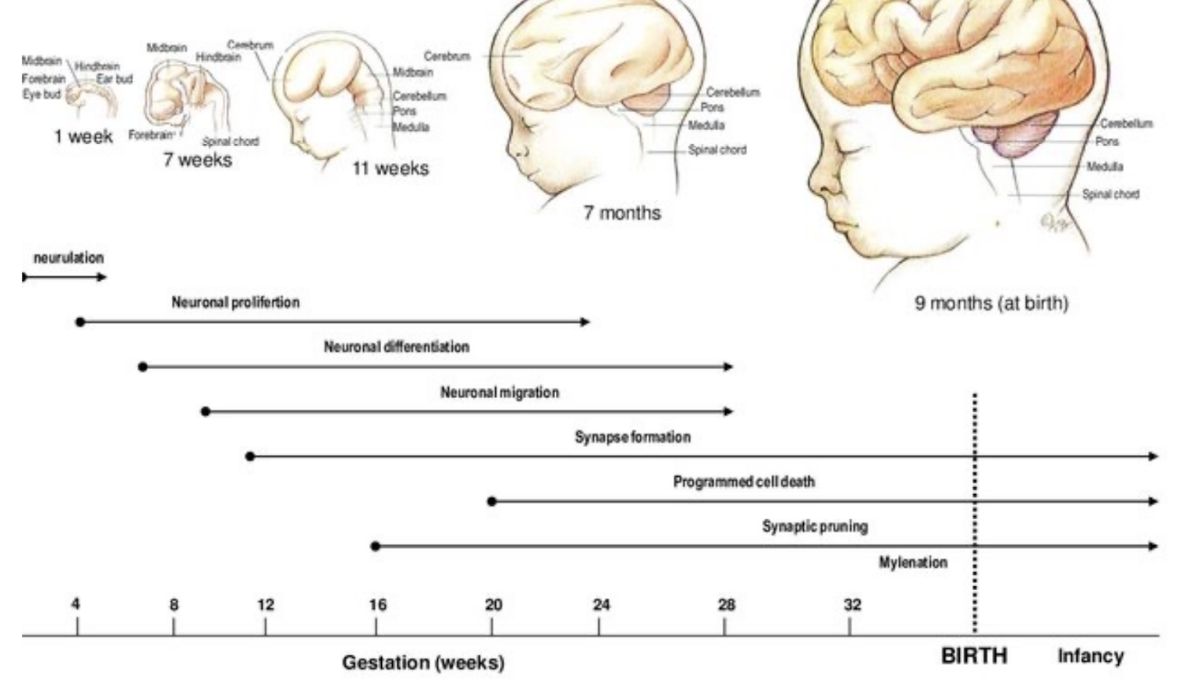
Image showing amount of synapses and connections over time (pre & post natally)
8 month premature, 1 month, 3 month, 6 month
15 month, 2 years, 4 years, 6 years
Between 2 & 6 synaptic pruning
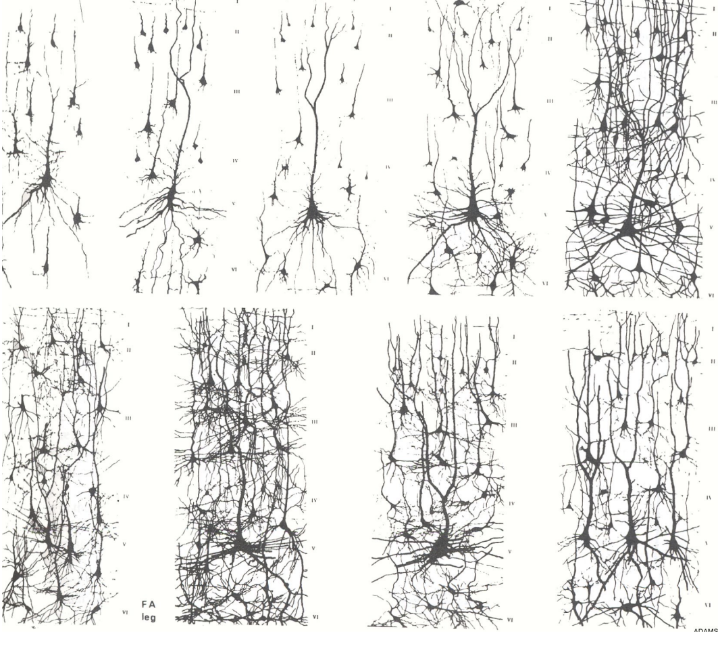
Myelination –
Due to schwann cells in PNS and oligodendrocytes in CNS –
In PNS all axons have coverage by schwann cells, only myelinated if cytoplasm of schwann cell wraps around axon more than once. (Each schwann cell only for 1 axon)
In CNS oligodendrocytes wrap themselves around more than one axon, which means there is more damage from loss of one oligodendrocyte than one schwann cell
Myelination over time
Myelination occurs not as much in fetal months (only upper in cortex), then in months of first year very much, and in 2-10 years lower down develops –
This shows up to down down corticospinal tract (cortex → spine)
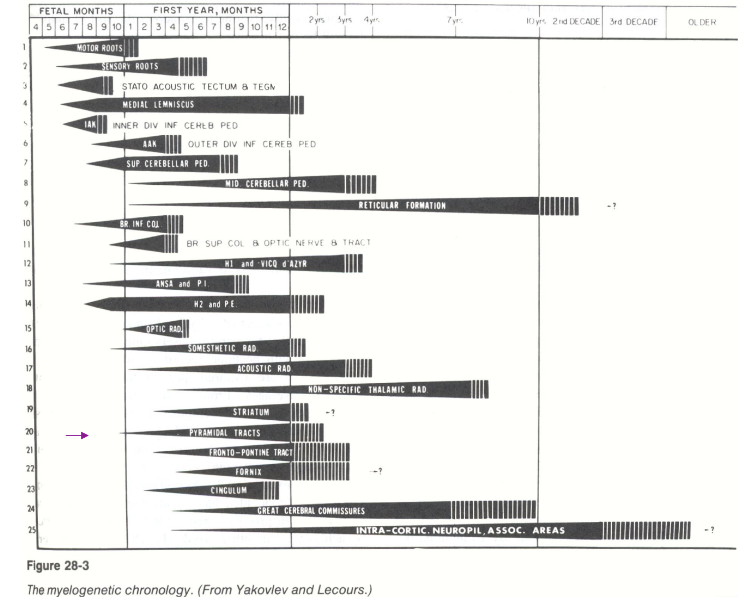
Babinski/Plantar reflex –
Normal reflex in infants after the sole of the foot has been firmly stroked – the big toe then moves upward or toward the top surface of the foot, the other toes fanning out.
Normal in children up to 2 years old, disappears as child gets older as early as 12 months – if doesn’t disappear could be indicator of some issue (damage to corticospinal tract)
OTHER REFLEXES ASWELL — not sure if need to know?
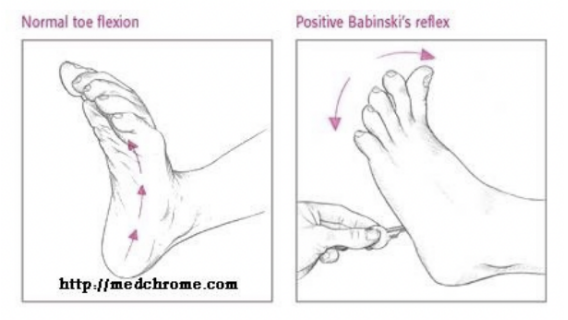
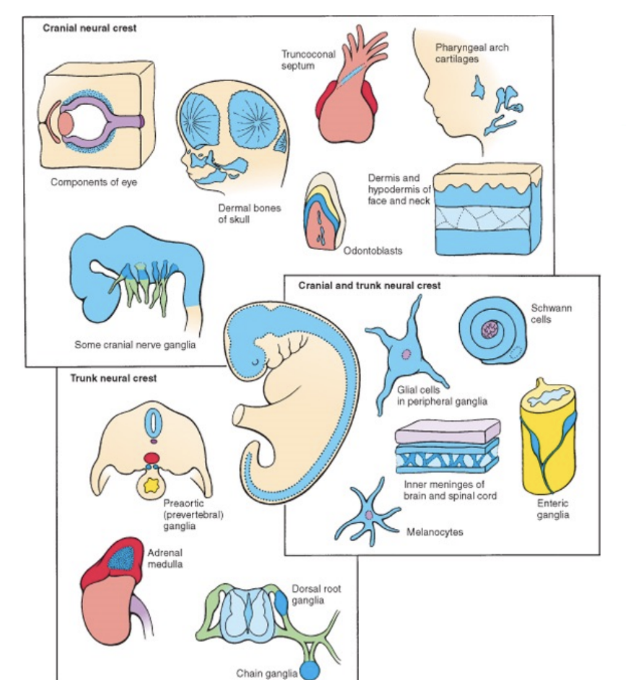
Derivatives of the neural crest & the placodes
The neural crest is the origin of the peripheral nervous system (PNS)
The portion of the nervous system not inside dorsal body cavities
Melanocytes not part of PNS but derives from neural crest
It also originates components of pharyngeal arches & thyroid parafollicular cells (not necessarily neural)
In order to give rise to these derivatives, they need to undergo an epithelial-mesenchymal transition in order to be able to delaminate from the other cells & migrate & colonize these areas
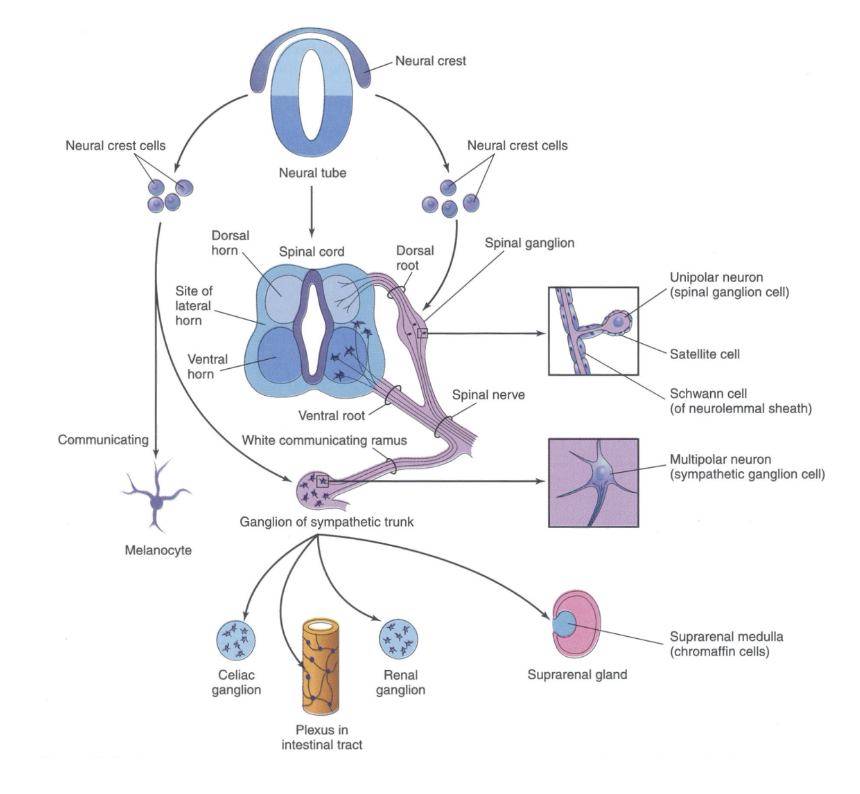
The neural crest can be divided into –
Cranial
Craniofacial bone & cartilage, Cranial neurons & glia, Odontoblasts, Melanocytes
Cardiac
Cardiac septa, Cardiac neurons & glia, Smooth muscle cells, Melanocytes
Vagal
Entering neurons & glia
Melanocytes
Trunk/sacral
Sensory neurons & glia
Autonomic neurons
Chromaffin cells
Melanocytes
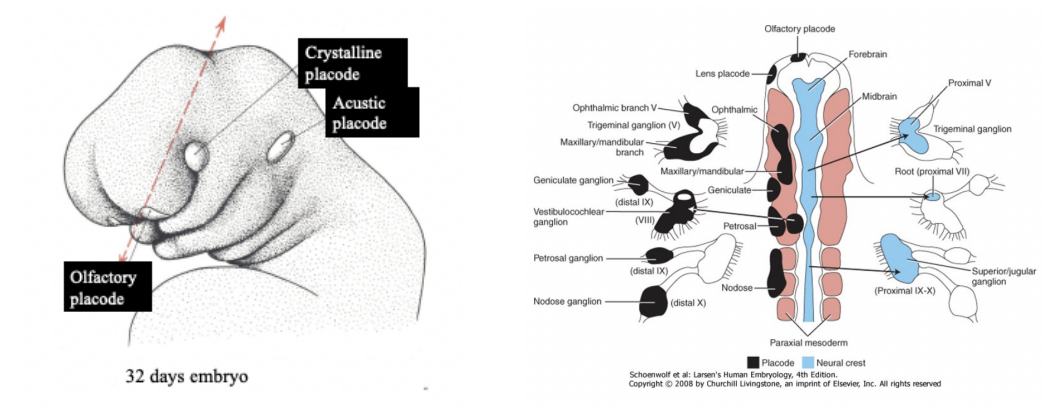
Placodes –
Localized ectodermal thickenings in the head of vertebrate embryos – involved in formation of sense organs (eye, nose, ear) & cranial sensory ganglia
Crystalline placode — eye
Acoustic placode — ear
Olfactory placode — nose
For formation of sensory ganglia of cranial nerves — contribution of neural crest cells aswell
Sensory ganglia of cranial nerves have a double origin –
Neural crest & placodes contribute to the formation of cranial nerves sensory ganglia
Neurocristopathies –
Class of pathologies occurring in vertebrates, especially in humans that result from –
Abnormal specification, migration, differentiation or death of neural crest cells during embryonic development.
Various pigment, skin, thyroid and hearing disorders, craniofacial and heart abnormalities, malfunctions of the digestive tract and tumors can also be considered as neurocristopathies.
Types —
Medullary carcinoma of the thyroid
Schwannoma
Neurofibromatosis Type I (von Recklinghausen disease)
CHARGE association –
Pheochromocytoma
Neuroblastoma
Cleft palate
DiGeorge Syndrome
Hirschsprung disease
Medullary carcinoma of the thyroid
Neurocristopathy —
Parafollicular cells, calcitonin
Sporadic (80%) or familial (20%) – can be associated with MEN (multiple endocrine neoplasia) – autosomal genetic disorder
Schwannoma
Neurocristopathy —
Benign tumour (PNS, schwann cells), most common: acoustic neurinoma
Neurofibromatosis Type I (von Recklinghausen disease)
Neurocristopathy —
Dominant genetic disorder, protein neurofibromin (tumorsuppressor gene): originates from Schwann cells
Multiple neural tumors (neurofibromas) dispersed in the body originating from peripheral nerves cells (neurites, fibroblasts, Schwann cells), pigmented skin lesions.
CHARGE association –
Neurocristopathy —
Coloboma of the retina, lens or choroid
Heart defects (e.g. Tetralogy of Fallot)
Atresia choanae
Retardation of growth
Genital abnormalities
Ear abnormalities or deafness
Pheochromocytoma
Neurocristopathy —
Generally in adrenal medulla, containing both epinephrine and norepinephrine.
Occurs between 40-60 years old and presents with persistent or paroxysmal hypertension, anxiety, tremor, profuse sweating, pallor, chest pain, and abdominal pain
Neuroblastoma
Common extracranial neoplasm, containing primitive neuroblasts
Mainly occurs in children (up to 15 years)
In sympathetic chain ganglia or in the adrenal medulla – presents wit hmetastasis to bones, bone marrow, and lymph nodes
Cleft palate, DiGeorge Syndrome, and Hirschsprung disease
Development of the spinal cord –
Useful prototype for studying the overall structural & functional features of the CNS
When we observe the neural tube at the spinal cord level we find the typical arrangement into 3 layers –
Marginal, mantle, and ventricular
In the wall of the neural tube at the level of the mantle layer (future grey matter) organizes into a dorsal/posterior territory and basal/anterior territory –
2 Alar plates
2 Basal plates
These plates will form the horns
Lumen becomes spinal/ependymal canal (becoming smaller) , and tiny lateral horns form
Gray matter divided into sensory & motor territories, and is all clustered together in dorsal & ventral area (thus easy division – clearly marked – in other places not as clear) —
Dorsal — sensory
Ventral — motor
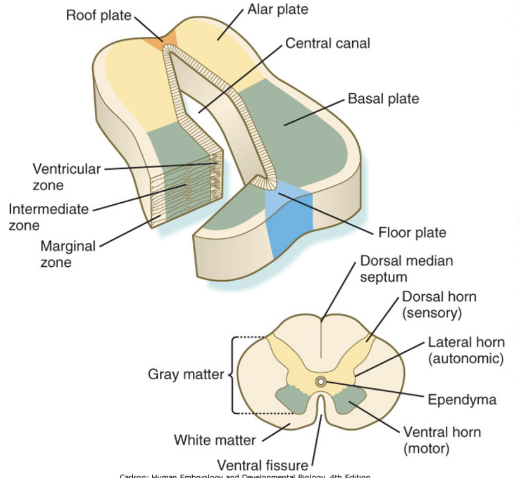
Alar & Basal plates
Dorsal/posterior territory and basal/anterior territory of wall of neural tube at level of mantle layer (future grey matter)
2 Alar plates
In between 2 alar plates – roof plate
Form sensory portion of neural tube
Non-permissive – axons cannot cross
2 Basal plates
In between 2 basal plates – floor plate
Form motor region of neural tube
Permissive – axons can cross
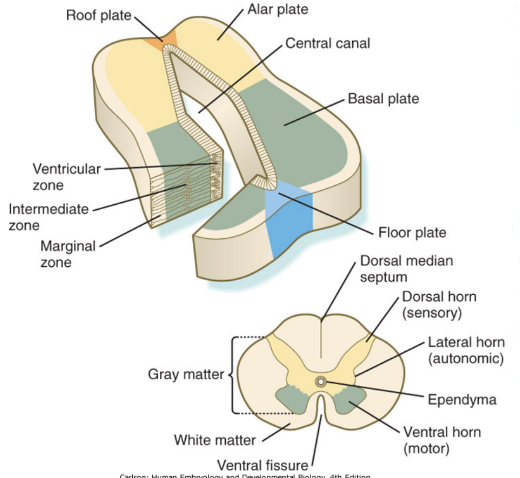
Gray matter division & patterning
Gray matter divided into sensory & motor territories, and is all clustered together in dorsal & ventral area (thus easy division – clearly marked – in other places not as clear)
Morphagens and transcription factors specify the dorso-ventral patterning of progenitors in the neural tube – specify what is where (motor ventral, sensory dorsal)
Opposite gradients of SHH and BMPs (bone morphogenic protein) determine the dorsal-ventral cell fates –
Floor plate, roof plate, etc produce morphogens, which are more concentrated either to the epidermis or the notochord, with these gradients triggering the transcription of transcription factors of Class I & II homeobox, etc
The notochord has influence on development of floor plate and exit sites of nerves from the spinal cord – issues with notochord will cause issues in neural tube development
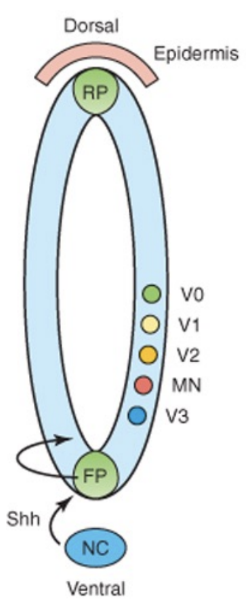
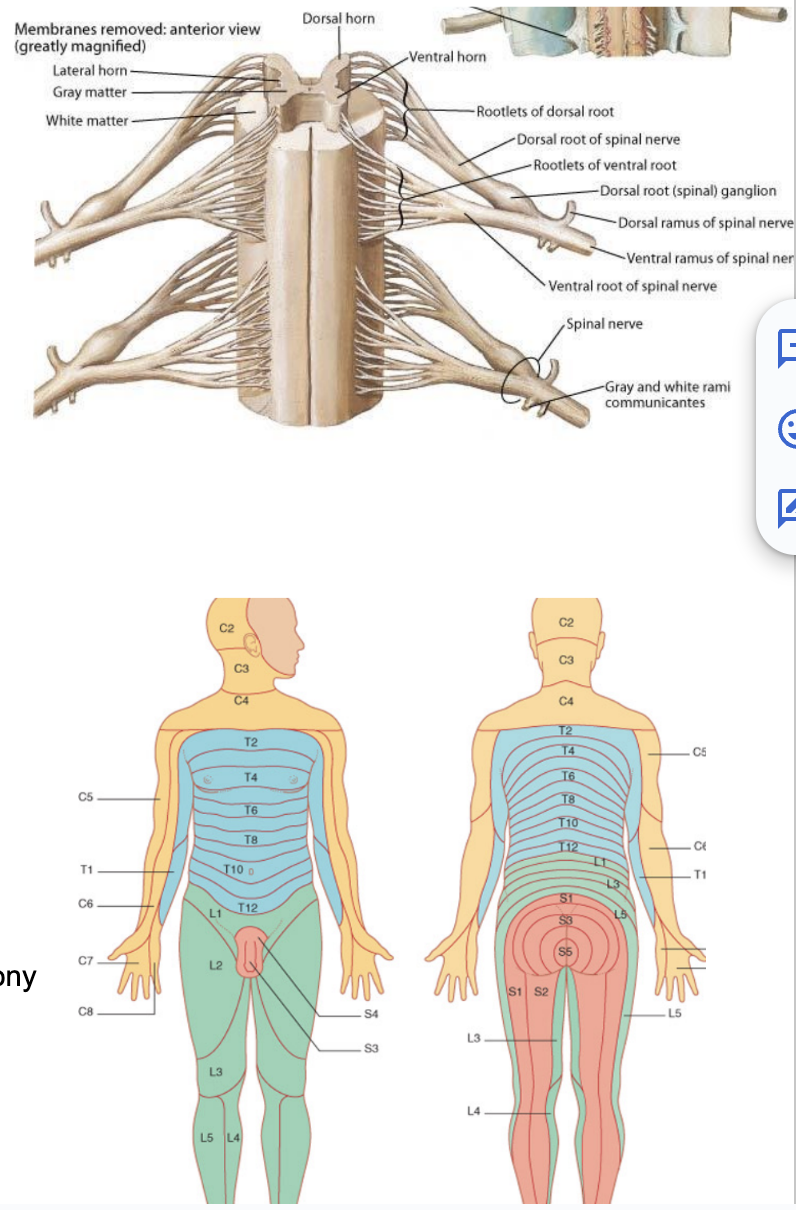
Development of spinal nerves (nerves originating from spine to periphery)
While alar & basal plate are forming, the neural crest cells on the side that didn’t migrate will form the sensory ganglia along with the placodes — forming the dorsal roots
Neurons going outside to periphery centrifugal, going inside from periphery is centripetal (referring to sensory ganglia but also all neurons in general)
Axons of centripetal sensory neurons go in from periphery reaching spinal cord from the dorsal root
Axons of centrifugal motor neurons go out to periphery from the ventral root (to the derivatives of a somite)
In basal plate — origination of motor neurons — innvervate & control muscle fibers originating from somites next to them
On each segment of the spinal cord on either side the dorsal root & ventral root come together to form a spinal nerve (spinal nerve = mixed)
2 spinal nerves per segment
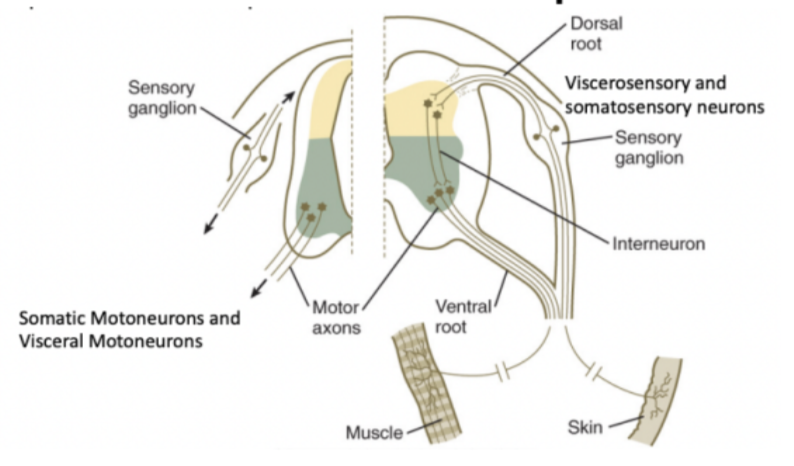
Each spinal nerve has 4 components –
Somatosensory
Territory receiving information from soma (skin, muscle, joints, ligaments, bones)
Viscerosensory
Neurons on the spinal cord receiving more visceral (organs) information
Visceromotor
Somatomotor
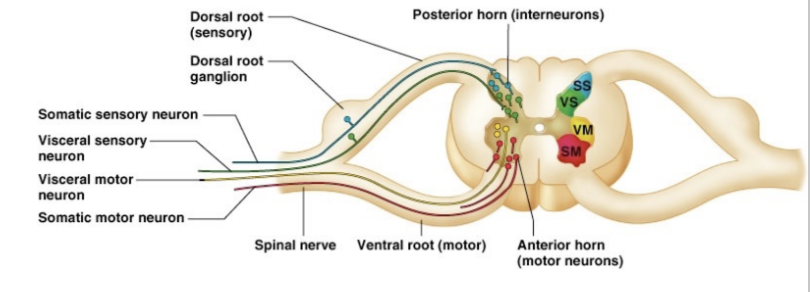
Describe the difference between somatomotor innervation and visceromotor innervation
Innervation of striated voluntary muscles vs innervation of smooth muscle
Visceral –
Preganglionic axon & autonomic/ganglionic neuron between neuron and target smooth muscle, so at minimum passes through two synapses
Visceromotor/preganglionic neuron in the CNS has an axon linked outside the CNS via a ventral root, then reaching other neurons on autonomic ganglia, finally innervating smooth muscle/glands in visceral organs
Somatic –
Directly connects to muscle
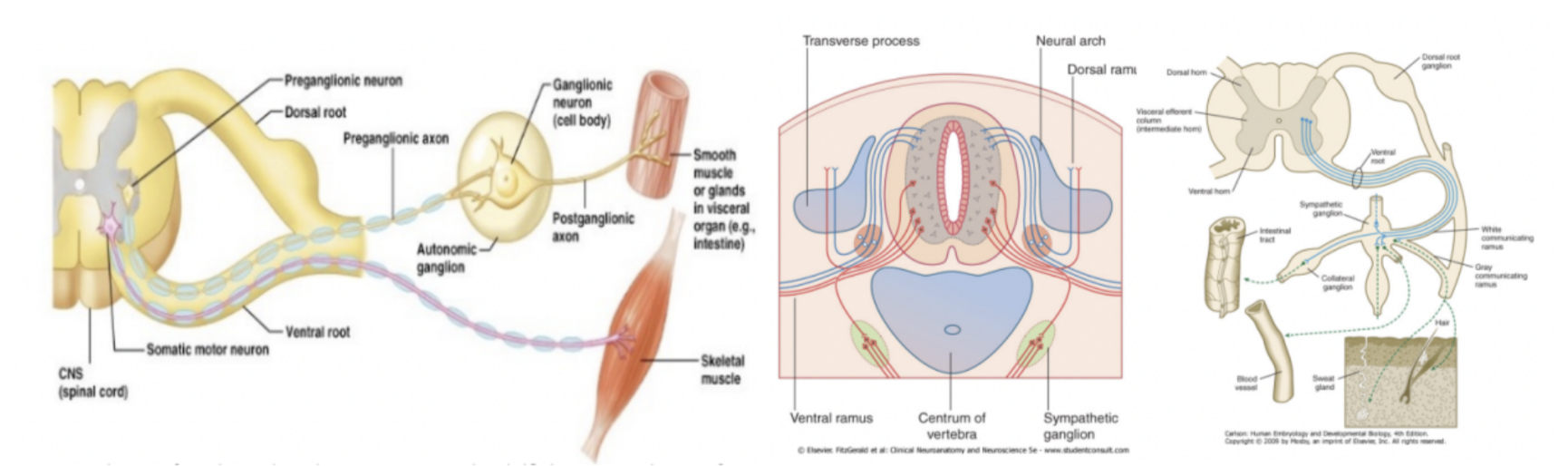
Segments of the spinal cord (+ dermatometric map)
The spinal cord is divided into segments (somitogenesis) – called neuromeres
Each segment contain
2 dorsal roots
2 ventral roots
2 spinal nerves (linkage of 1 dorsal & 1 ventral root)
Each segment innervates the cutaneous territory (dermatone), bony territory (sclerotome), and the muscular territory (myotome) originating from the adjacent somite
This connection is maintained even when somites disappear – dermatomeric map – displays stretches of skin whose sensory innervation depends mostly from a single segment of the spinal cord
Innervation and motor control of most muscles depends on more than 1 segment of the spinal cord — not as clear as bone/skin
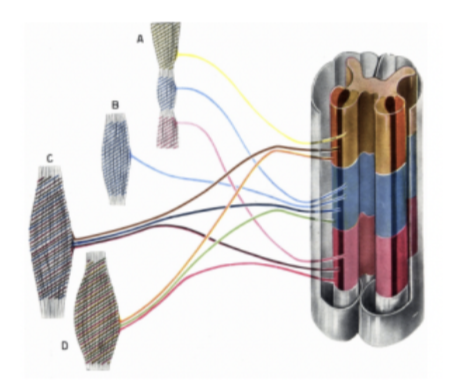
Spinal (/neural) plexuses
As a neuromere usually innervates more than one muscle, and one muscle is innervated by more than one neuromere — formation of anastomosis between nerves of different neuromeres — thus forming neural plexuses — bundles of intersecting nerves
Most spinal nerves (exception — thoracic) form spinal plexuses to exchange fibers with one another
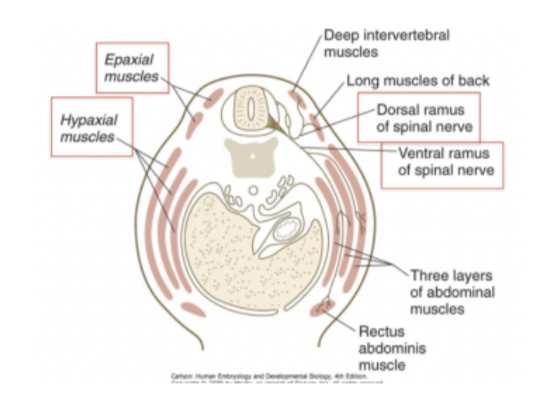
Dorsal & ventral rami of spinal nerves
Rami=branches – not roots
Some muscles that spinal nerves need to innervate are ventral while others are more posterior (rostral?) — thus each spinal nerve divides into into a dorsal & ventral ramus/branch (very early on in development)
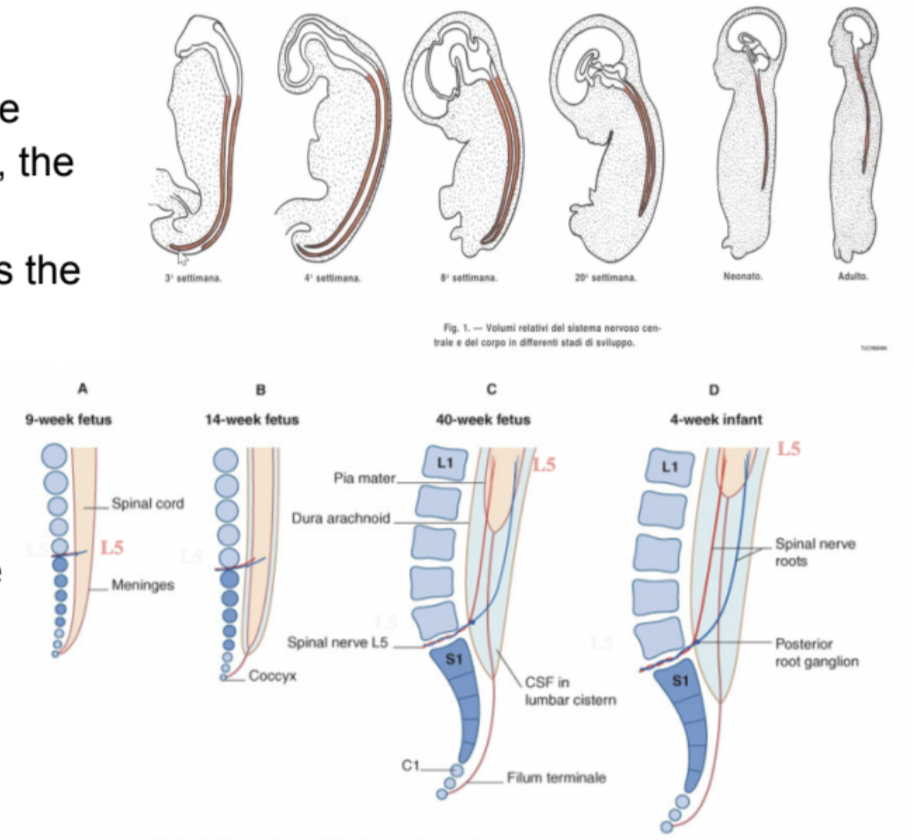
Ascent of the spinal cord –
Ventral & dorsal roots leave the vertebral canal caudal to the vertebrae with the corresponding number
Meaning that those axons begin to make connections with the periphery
But then, the vertebral column begins to elongate further, leading the roots to elongate or lose the connection with the periphery
Nerve roots acquire a progressively more oblique course in the vertebral canal – the more developed the more oblique – postnatally appear almost straight