Group 7 PMT (q. 1-16)
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms

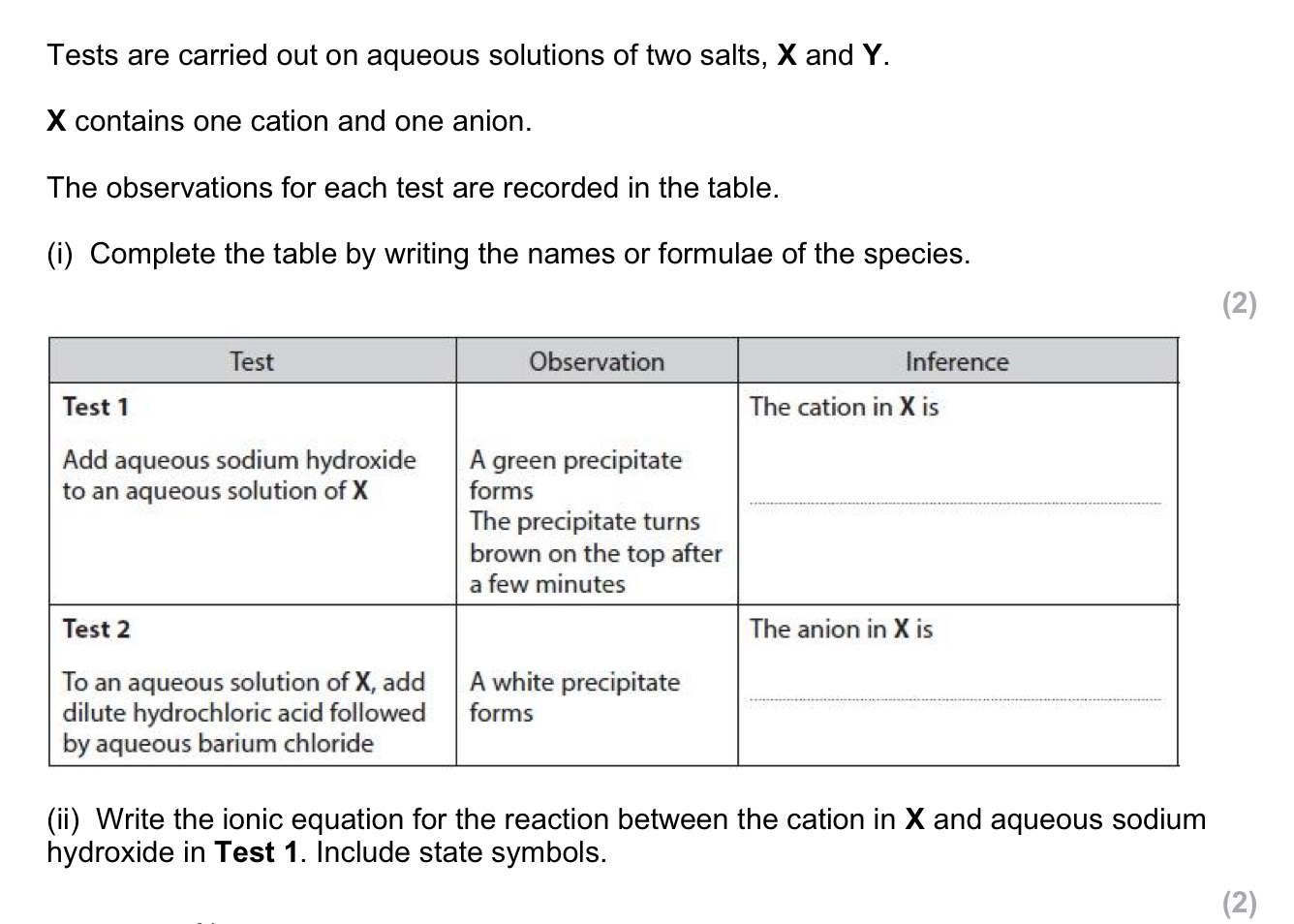
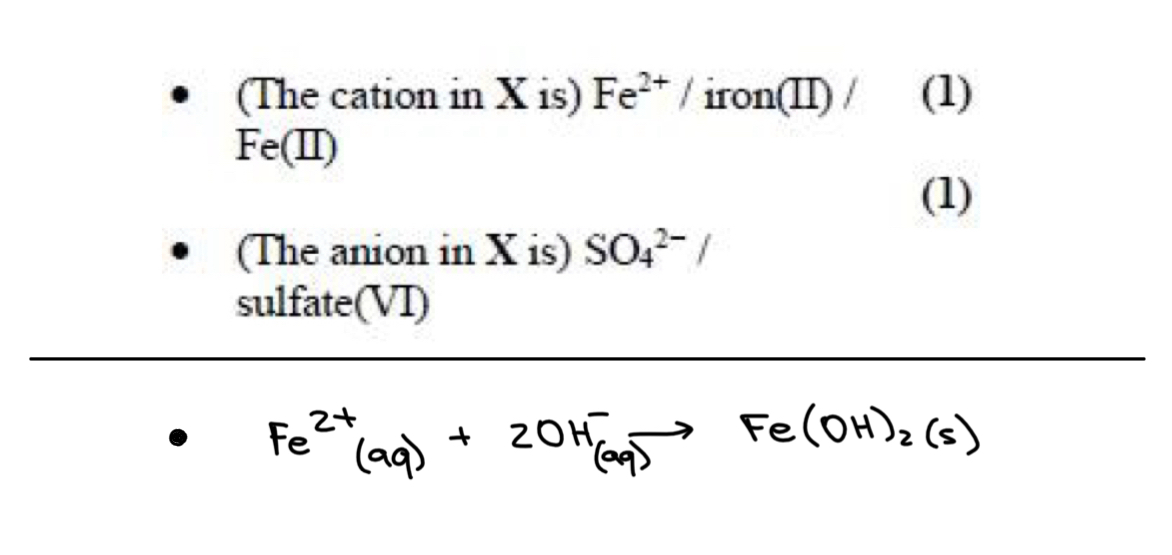
add HCl + BaCl2
white ppt
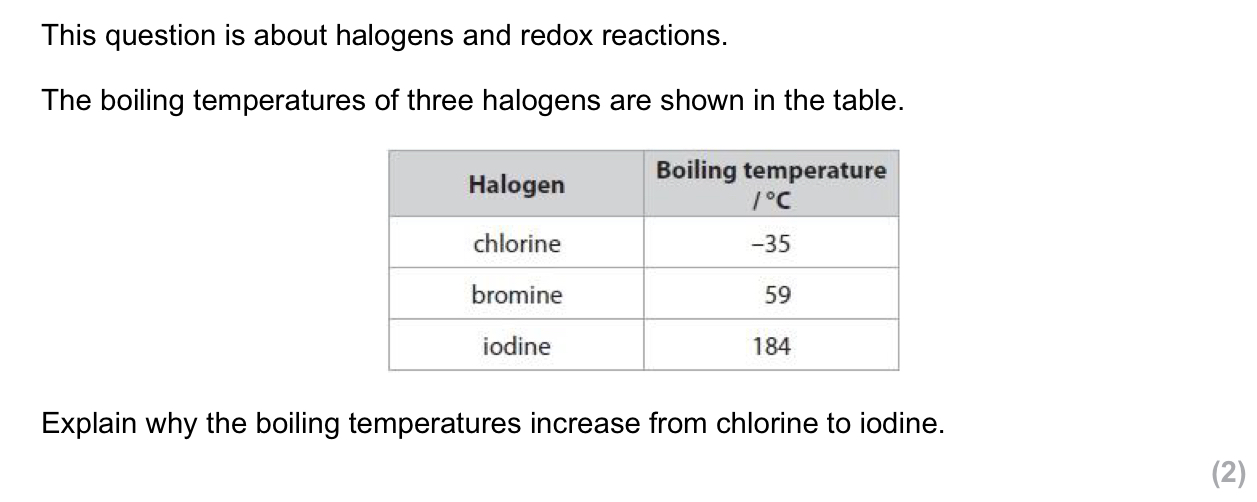
no. of electrons increases down the group- from chlorine to iodine
the strength of the London forces increases and more energy is needed to overcome the London forces
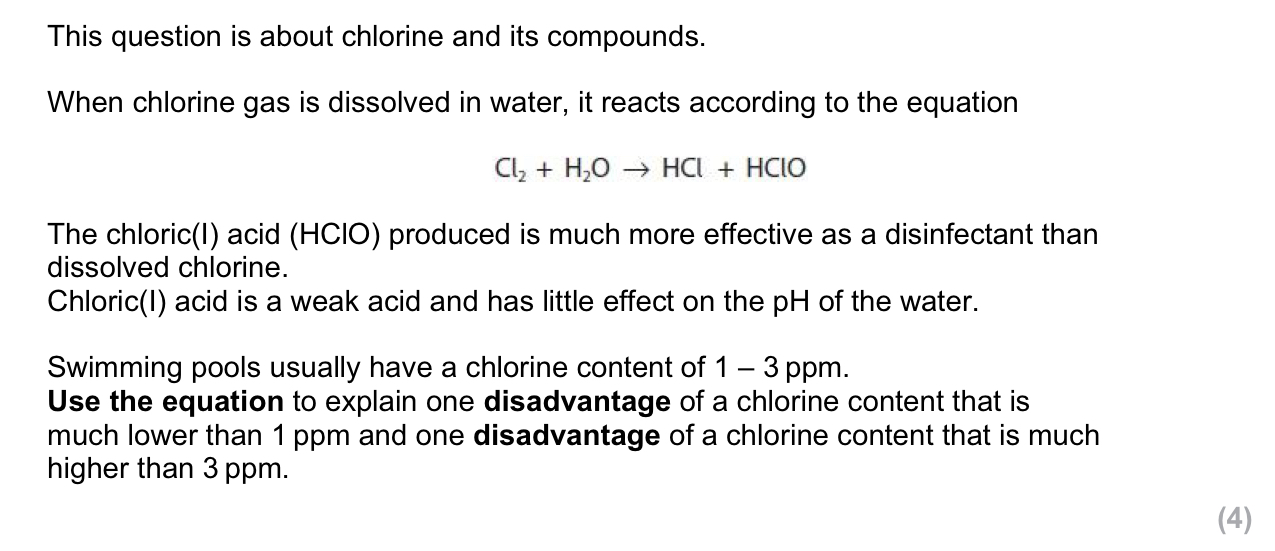
lower:
HClO will be lower
ineffective as a disinfectant
higher:
HCl will be higher
increases toxicity/ acidity

excess dilute ammonia and the ppt is insoluble
excess conc. ammonia and it is soluble
Give a reason why dilute hydrochloric acid is needed to test for sulfate ions?
To remove any carbonate/ sulfite
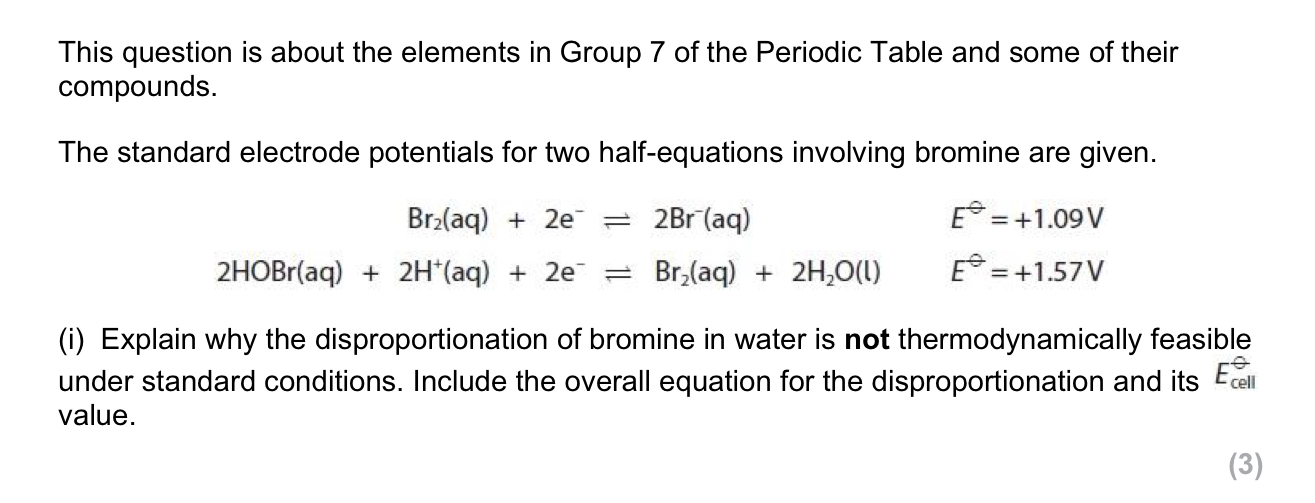
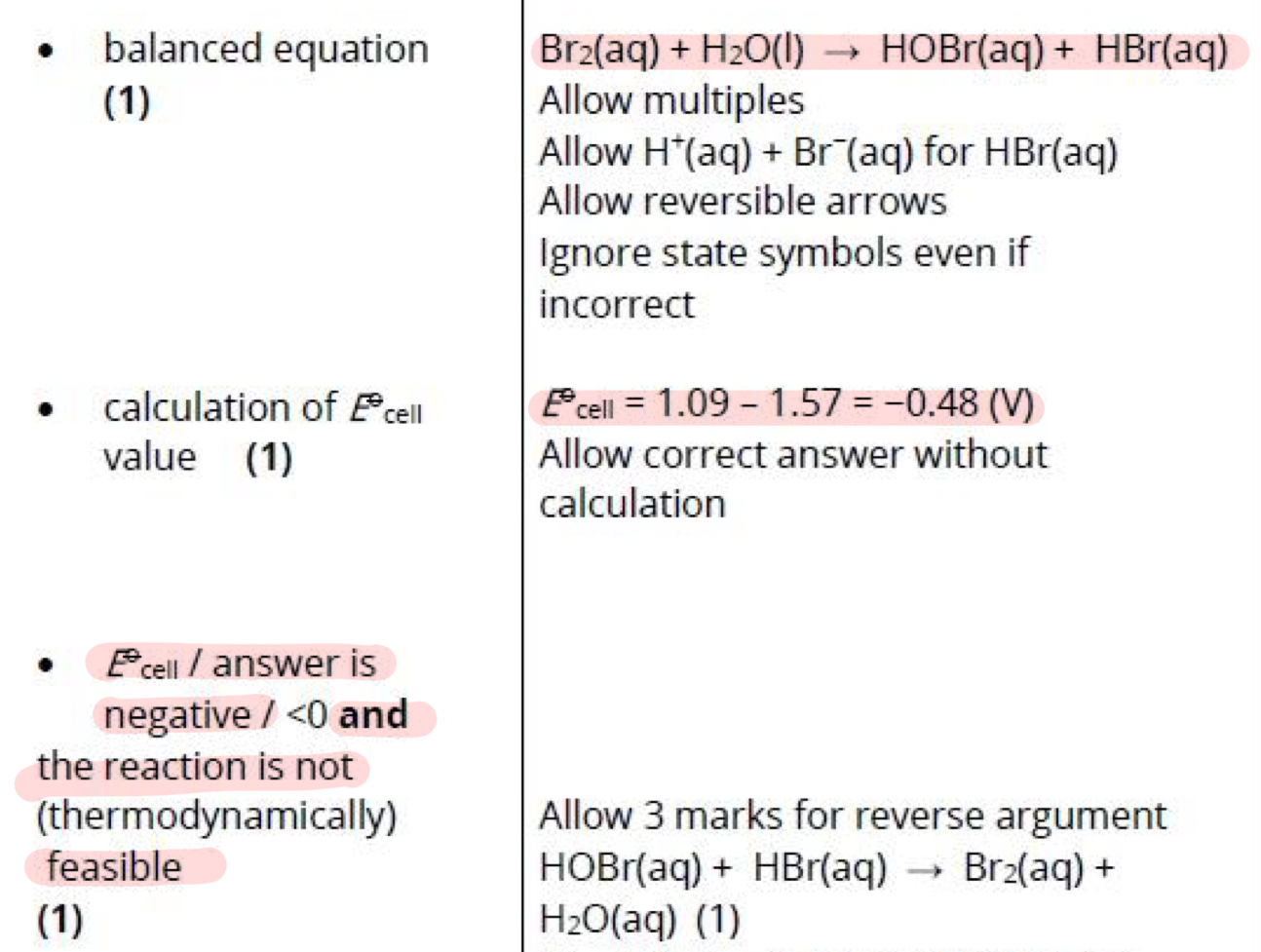
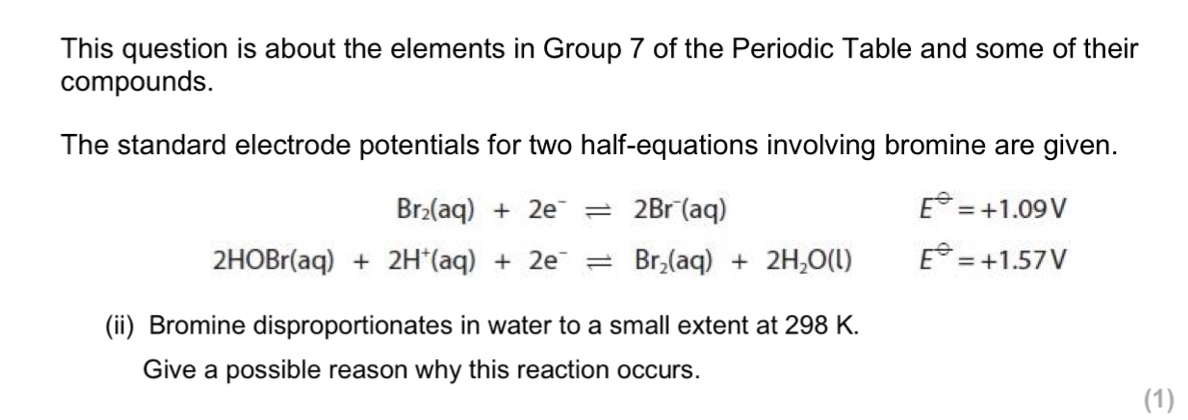
Disproportionation is an equilibrium system
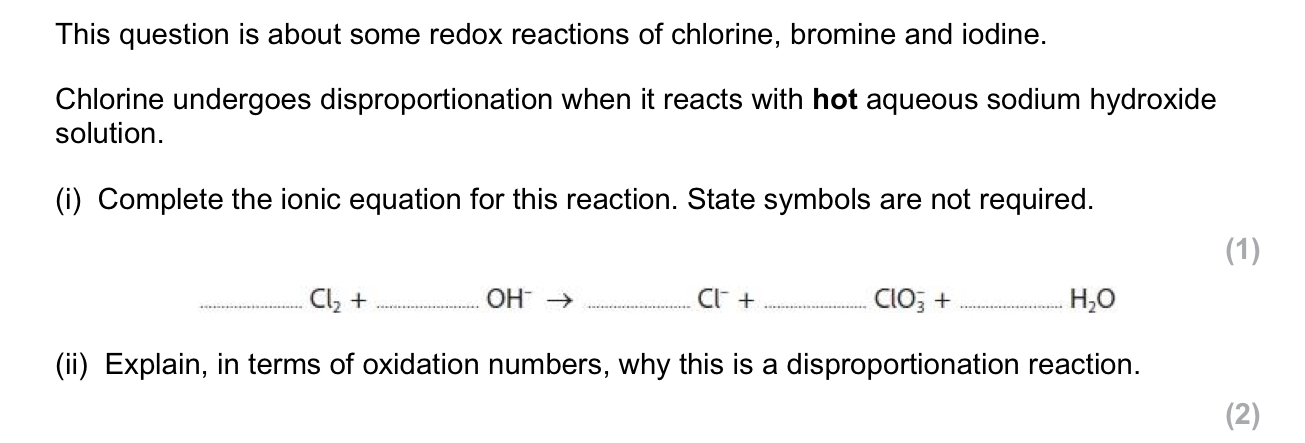
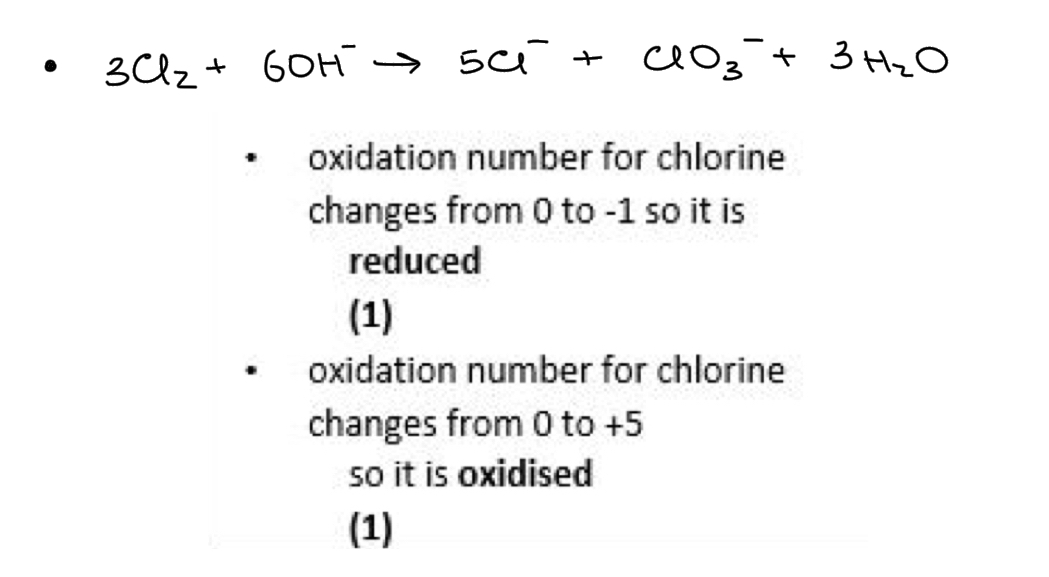
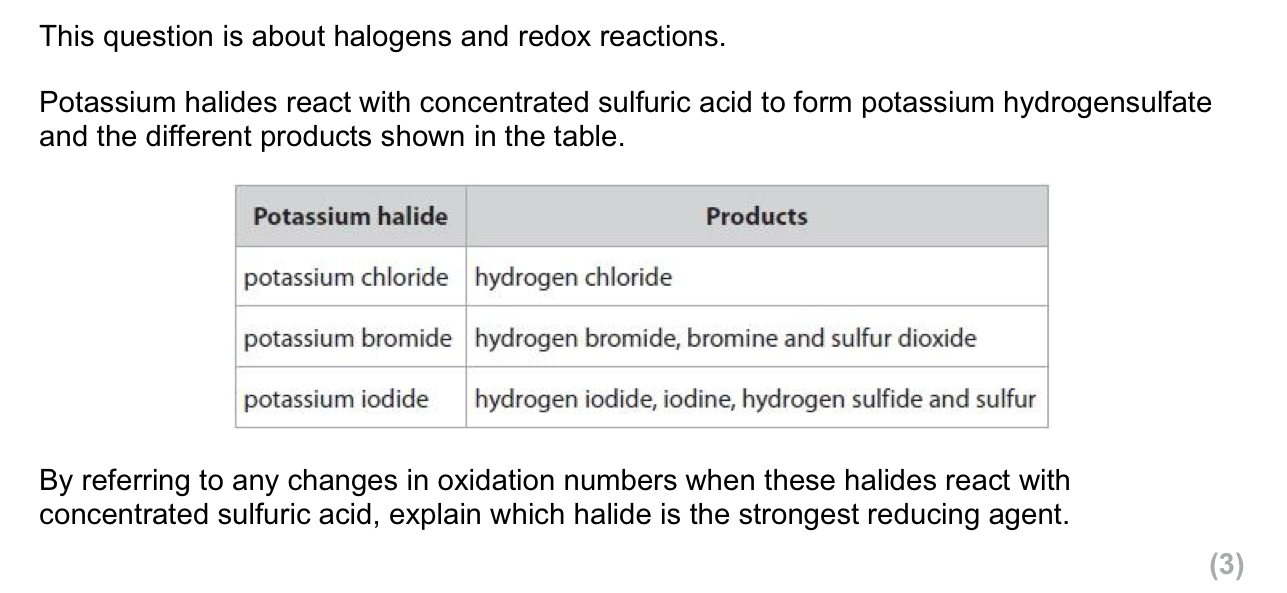


The covalent bond in HCl changes to an ionic bond in aqueous solution

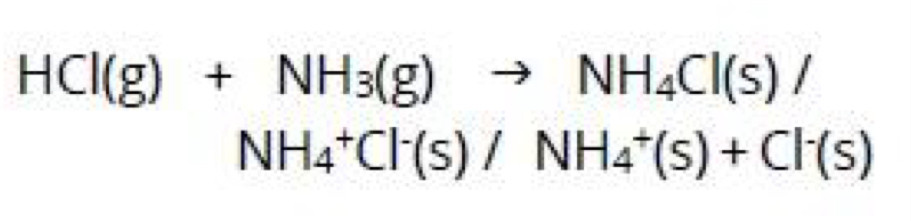



NH4Br
Write the ionic equation for the reaction between ammonium cation and sodium hydroxide producing the gas
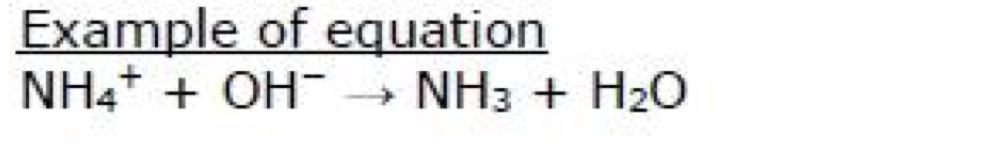

Write the ionic equation for the reaction between aqueous solutions of sodium iodide and silver nitrate. Include state symbols
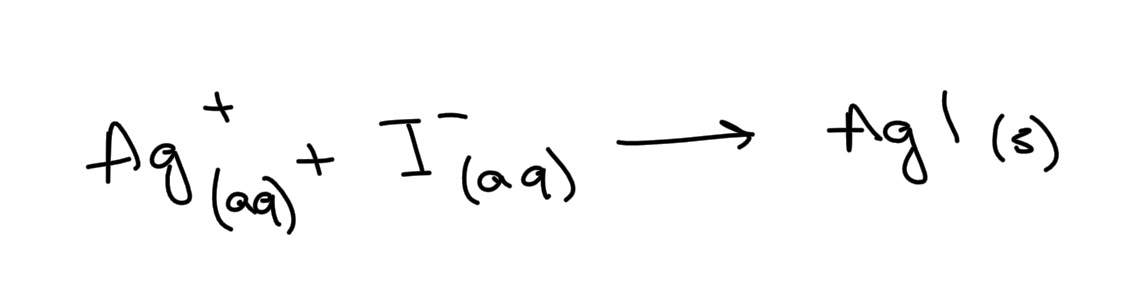
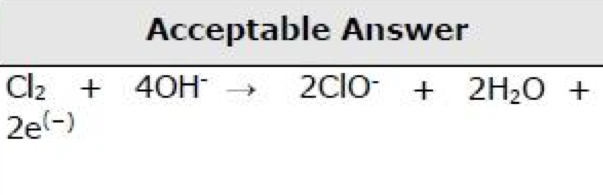
Ionic half-equation for the reduction of chlorine molecules to chloride ions.

Ionic half-equation for the oxidation of chlorine molecules to chlorate(I) ions in the presence of cold, aqueous hydroxide ions