Valence Electrons - Chemistry
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
ion
When an atom loses electrons or gains electrons.
octet rule
What is the rule of how an atom can achieve a stable electron configuration?
Anion
when an ion gains electrons, it is…
Cation
when an ion loses electrons, it becomes a…
positive
If an atom loses electrons, the charge will be…
negative
If an atom gains electrons, the charge will be…
Noble Gas
This can’t form into an ion because it already has 8 valence electrons
Carbon and Silicon
This can’t form into an ion because it has 4 valence electrons
Boron
This can’t form into an ion because it has 3 valence electrons but can’t form into an ion easily.
H+
Ion of H
Be2+
Ion of Be
B3+
Ion of B
N3-
Ion of N
O2-
Ion of O
F1-
Ion of F
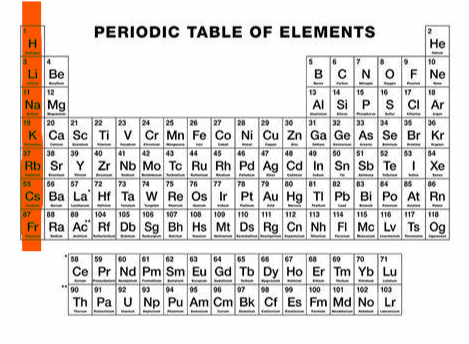
+1 (group 1)
What charge and group is this?
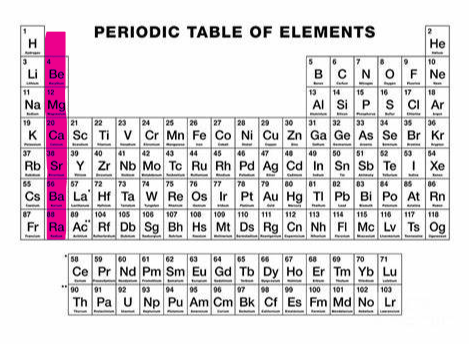
+2 (group 2)
What charge and group is this?
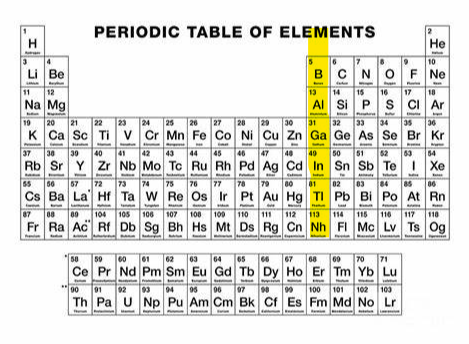
+3 (group 13)
What charge and group is this?
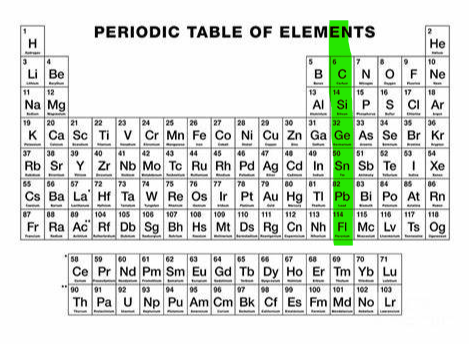
+4,-4 (group 14)
What charge and group is this?
-3 (group 15)
What charge and group is this?
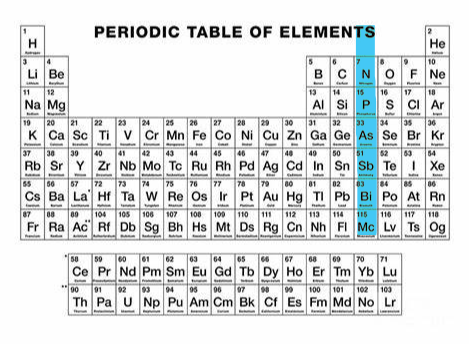
-2 (group 16)
What charge and group is this?
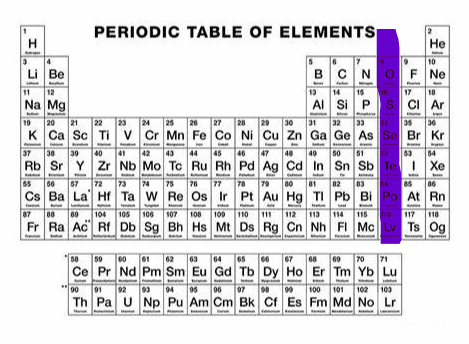
-1 (group 17)
What charge and group is this?
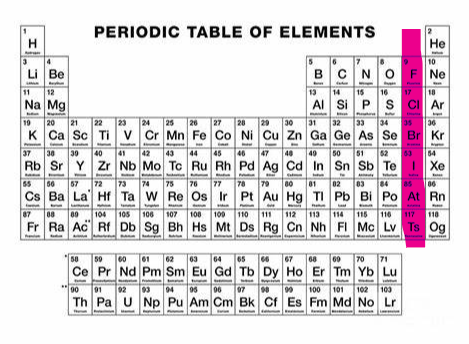
Neutral (Noble Gases) (group 18)
What charge and group is this?
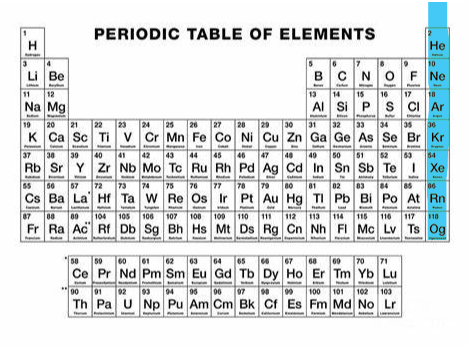
Alkali metal
Group 1
Alkali earth metal
Group 2
other metals
Group 13
Transition metals
Group 3-12
Other Non-metals
Group 14
Other non-metals
Group 15
Other non-metals
Group 16
other Halogens
Group 17
Nobel gas
Group 18