Properties of Water
5.0(3)
Card Sorting
1/24
There's no tags or description
Looks like no tags are added yet.
Last updated 5:56 PM on 9/13/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
1
New cards
The Process of Life Beginning in Water
* first cells and their membranes formed around small volumes of water
* chemical reactions happen between water dissolved substances
* liquid water allows molecules to interact
* chemical reactions happen between water dissolved substances
* liquid water allows molecules to interact
2
New cards
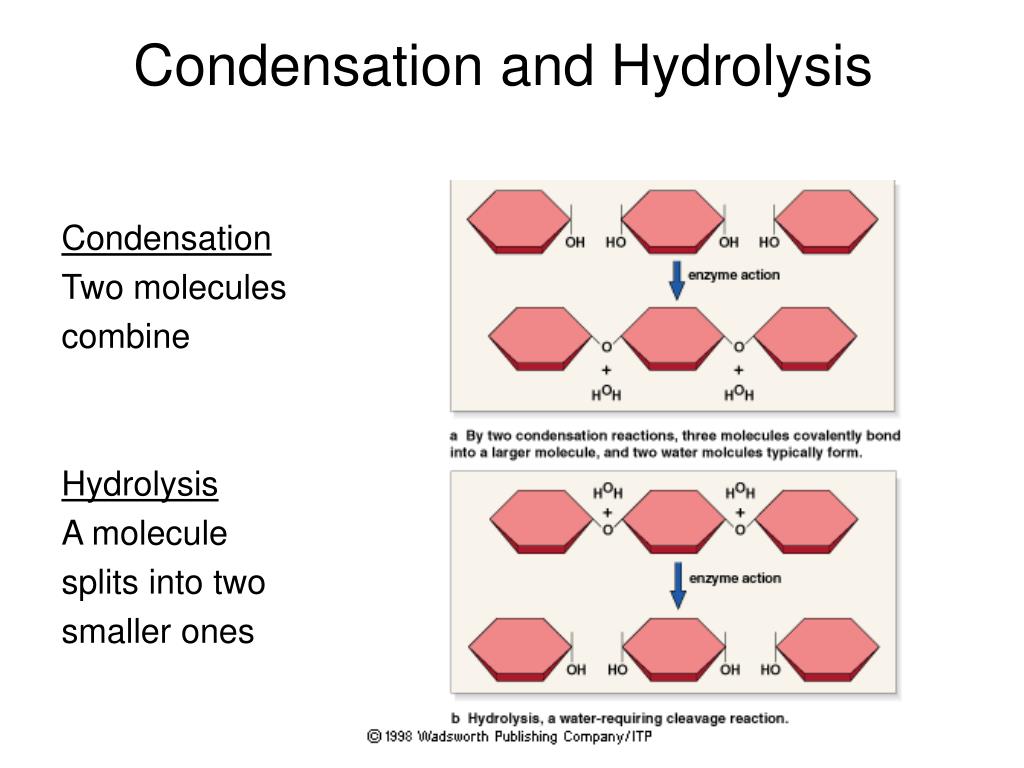
condensation reaction
water formed as one of the products when two molecules join together
3
New cards
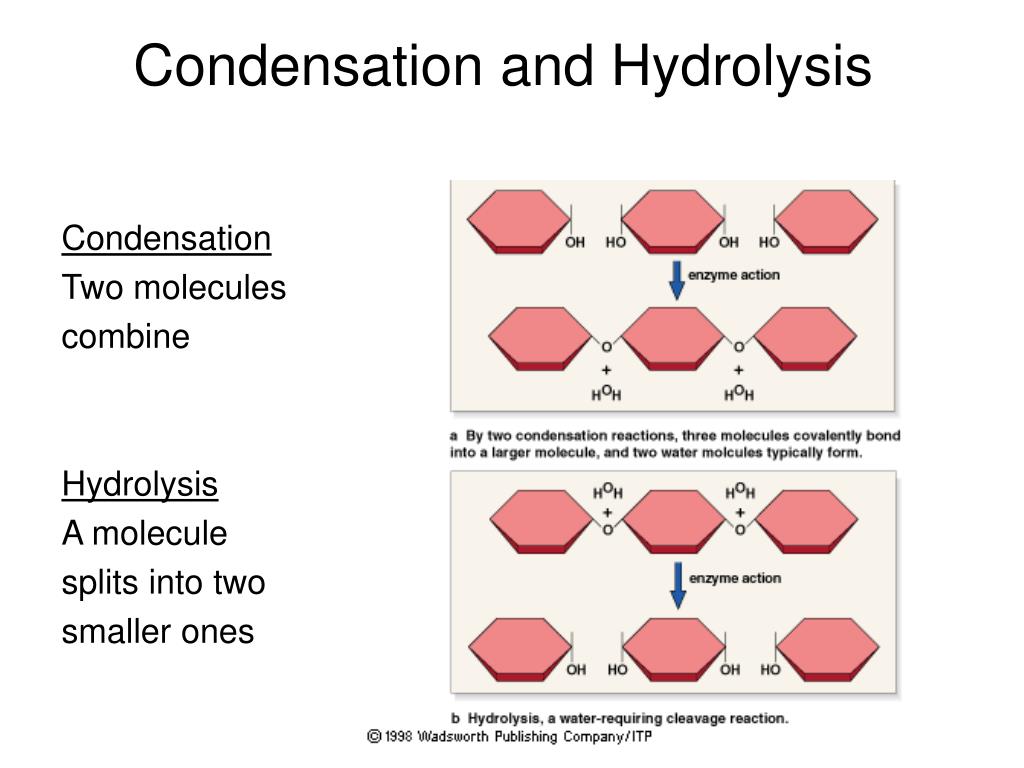
hydrolysis reaction
water reacts with chemical to break into smaller molecules
4
New cards
metabolism
chemical reactions occuring in cells
5
New cards
metabolites
chemicals involved in metabolism are
6
New cards
water is a metabolite
either as reactant or product
7
New cards
metabolic reactions in cells are catalyzed by enzymes →
which can be impacted by temperature
8
New cards
what buffers temperature and why?
water due to high specific heat capacity
9
New cards
Examples of Water maintaining biological structures
* water contributes to the formation of cell membranes
* water impacts the folding of proteins
* water surrounds DNA to support the double-helix
* water impacts the folding of proteins
* water surrounds DNA to support the double-helix
10
New cards
the atom is the
smallest unit of matter.
11
New cards
atoms are composed of
protons(+), neutrons(no net charge), electrons(-)
12
New cards
atoms are electrically neutral if they have
equal protons and electrons.
13
New cards
ion
atom with charge
14
New cards
atoms form bonds through
electrons transferring to other atoms or being shared
15
New cards
chemical bond →
attraction between atoms, ions, molecules forming chemical compounds
16
New cards
types of bonds
polar or nonpolar covalent, ionic, hydrogen
17
New cards
covalent bonds hold two atoms together by
sharing two or more electrons
18
New cards
non polar covalent
equal sharing of electrons
19
New cards
polar covalent
unequal sharing of electrons
20
New cards
ionic bond
attraction between cation(+) and anion(-). electron donated to nonmetal atom
21
New cards
hydrogen bonds
attractions between two polar molecules
22
New cards
polar molecule
one end is slightly positive and one end is slightly negative
23
New cards
water molecules have
* 2 hydrogen and 1 oxygen bonds
* electrons shared through polar covalent bonds between atoms
* oxygen atom has more protons so attracts the shared electron more
* electrons shared through polar covalent bonds between atoms
* oxygen atom has more protons so attracts the shared electron more
24
New cards
multiple water molecules
partial positives on the hydrogen bonds of one water molecule are attracted to partially negative oxygen atom of DIFFERENT water molecule → forms a hydrogen bond
25
New cards
water can have up to
4 H bonds