Science Test 1
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
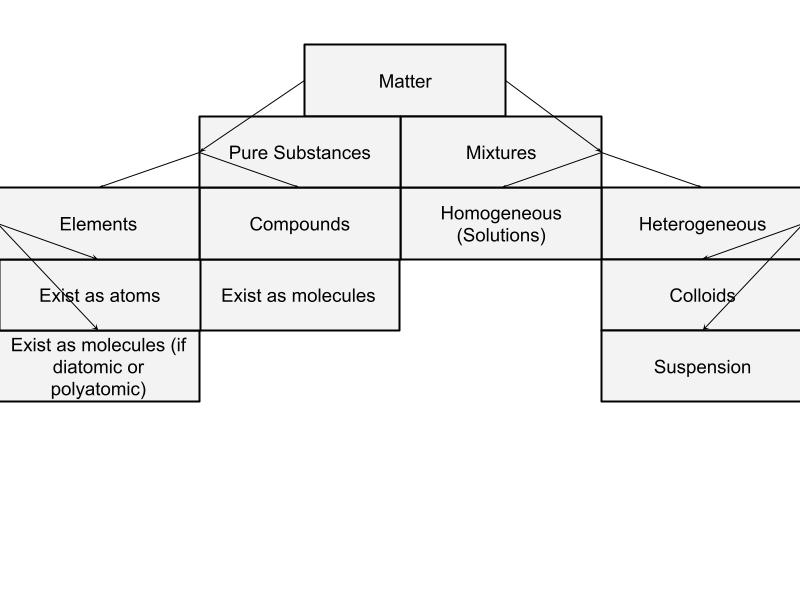
Suspension
A mixture in which the particles of a substance are mixed throughout but are large enough to settle
Colloid
A mixture in which the particles of a substance are dispersed throughout and are not large enough to settle
Physics
The study of matter, energy, motion, and forces
Chemistry
The study of how atoms, molecules, and chemical interact with each other
Atom
The smallest unit into which an element can be divided and still maintain its properties
Molecule
The smallest unit into which an element can be divided and still maintain its properties
Heterogeneous Mixture
A combination of substances in which the particles are not uniformly distributed
Matter
Anything that has mass and takes up space
Mixture
A combination of two or more substances that are not chemically combined
Tyndall EffectB
The scattering of light by colloids
Benefits of Physics
medicines, machinery, better foods, those are transportation, and more rapid communication methods
Drawbacks of Physical Science
Air and water pollution, radioactive waste, and other harmful environmental effects
Benefits of Chemistry
Plastics, pesticides, fuels, fabrics, paper, fertilizers, rubber, allies, and drugs
Characteristics of Suspensions
Can block light
Can be separated using filtration
Particles are large enough to settle
Examples of Suspensions
Salad dressing
Clay and water
Paint
Flour and water
Liquid antibiotics
Sunscreen
Red blood cells
Characteristics of Compounds
Two or more atoms chemically combined in a fixed proportion
Examples of Compounds
Water (H2O)
Hydrogen peroxide ( H2O2)
Salt (NaCl)
Characteristics of Homogeneous Mixtures
Properties and appearance are the same throughout
Examples of Homogeneous Mixtures
Sugar in hot water
Gold jewelry
Chromatography
A method of separating mixtures according to how the different components are absorbed
Distillation
A method of separating a liquid mixture by heating it so the liquid evaporates and condenses
Filtration
The use of paper or other materials to remove solid particles from a liquid mixture
Matter Breakdown