Chemistry 1 - Molecular Structure & Polarity
1/12
Earn XP
Description and Tags
Everything from electron groups, electron bonds/lone pairs, geometric names, molecular names, angles and its polarity
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
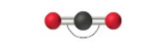
sp, 2 groups, 2 bonding, 0 lone pair NONPOLAR
Linear 180
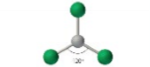
sp2, 3 groups, 3 bonding, 0 lone pair
Trigonal Planar 120
sp2 3 groups, 2 bonding, 1 lone pair
Trigonal Planar, Bent <120
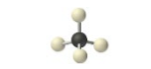
sp3, 4 groups, 4 bonding, 0 lone pair NONPOLAR
Tetrahedral 109.5
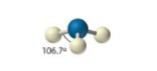
sp3, 4 groups, 3 bonding, 1 lone pair
Tetrahedral, Trigonal pyramid <109.5
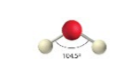
sp3, 4 groups, 2 bonding, 2 lone pairs
Tetrahedral, Bent <109.5
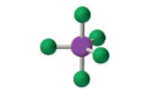
sp3d, 5 groups, 5 bonding, 0 lone pairs NONPOLAR
Trigonal Bipyramidal 120, 90 (axial)
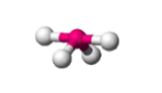
sp3d, 5 groups, 4 bonding, 1 lone pair
Trigonal Bipyramidal, Seesaw <120, <90 (axial)
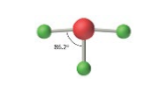
sp3d, 5 groups, 3 bonding, 2 lone pairs
Trigonal Bipyramidal, T-Shaped <90

sp3d, 5 groups, 2 bonding, 3 lone pairs
Trigonal Bipyramidal, Linear 180
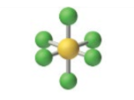
sp3d2, 6 groups, 6 bonding, 0 lone pairs NONPOLAR
Octahedral 90
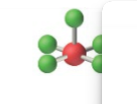
sp3d2, 6 groups, 5 bonding, 1 lone pair
Octahedral, Square pyramidal <90
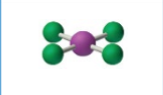
sp3d2, 6 groups, 4 bonding, 2 lone pairs
Octahedral, Square Planar 90