Miles Down Organic Chemistry
1/224
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
225 Terms
Number of Carbons: 1
Methane CH4
Number of Carbons: 2
Ethane C2H6
Number of Carbons: 3
Propane C3H8
Number of Carbons: 4
Butane C4H10
Number of Carbons: 5
Pentane C5H12
Number of Carbons: 6
Hexane C6H14
Number of Carbons: 7
Heptane C7H16
Number of Carbons: 8
Octane C8H18
Number of Carbons: 9
Nonane C9H20
Number of Carbons: 10
Decane C10H22
IUPAC Naming Convention Steps
Step 1: Find the parent chain, the longest carbon chain that contains the highest-priority functional group
Step 2: Number the chain in such a way that the highest-priority functional group receives the lowest possible numbers
Step 3: Name the substituents with a prefix. Multiple of the same type receive di-, tri-, tetra, etc.
Step 4: Assign a number to each substituent depending on the carbon to which it is bonded
Step 5: Alphabetize substituents and separate number from each other by command and form words by hyphens
Alkane
Saturated hydrocarbon with no double or triple bonds CnH2n+2
Naming: Named according to the number of carbons present following by the suffix -ane

Isopropyl
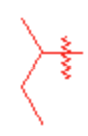
Sec-butyl

Tert-butyl
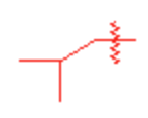
Isobutyl
Alkene
Contains a double bond. Use suffix -ene
Alkyne
Contains a triple bond. Use suffix -yne
Alcohol
Contains a -OH group. Use suffix -ol or prefix hydroxy-
Have higher priority than double or triple bonds
Diol
Contains 2 hydroxyl groups
Geminal: If on same carbon
Vicinal: If on adjacent carbons
Carbonyl Group
C=O
Aldehydes and ketones both have a carbonyl group
The reactivity of a carbonyl is dictated by the polarity of the double bond
The carbon has a δ+ so it is electrophilic
Carbonyl containing compounds have a ↑ BP than equivalent alkanes due to dipole interactions
Alcohols have ↑ BP than carbonyls due to hydrogen bonding
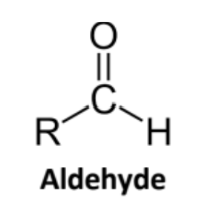
Aldehyde
Carbonyl group on terminal C, bonded to at least one H
Suffix -al
In rings: suffix -carbaldehyde
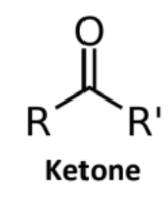
Ketone
Carbonyl group on nonterminal C, bonded to two alkyl chains
Suffix -one
Prefix oxo- or keto-
Are less reactive toward nucleophiles because of steric hindrance and ⍺-carbanion de-stabilization
The presence of an additional alkyl group crowds the transition steps and increased energy
The alkyl group also donates e- density to the carbanion, making it less stable
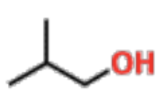
Primary Alcohols
C attached to OH is only attached to 1 other C
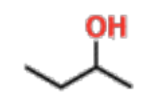
Secondary Alcohols
C attached to OH is attached to 2 other Cs
Tertiary Alcohols
C attached to OH is attached to 3 other Cs

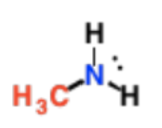
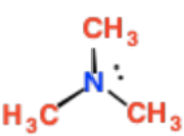
Primary Amines
N is only attached to 1 C
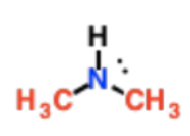
Secondary Amines
N is attached to 2 Cs
Tertiary Amines
N is attached to 3 Cs
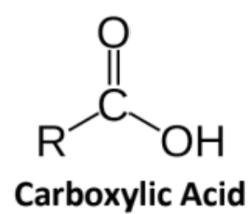
Carboxylic Acid
The highest priority functional group because it contains 3 bonds to oxygen
Suffix “-ioc acid”; Salts: -oate; Dicarboxylic Acids: -dioic acids
Always terminal groups
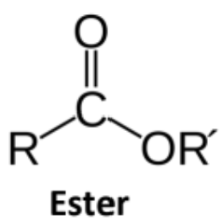
Ester
Carboxylic Acid derivative where -OH is replaced with -OR
The condensation products of carboxylic acids with alcohols
Given the suffix -oate
The esterifying group is written as a substituent, without a number
Cyclic esters = lactones; named by the number of carbons in the ring and the Greek letter of the carbon forming the bond with the oxygen
Triacylglycerols include three ester bonds between glycerol and fatty acids
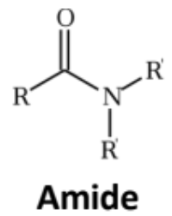
Amide
Replace the -OH group of a carboxylic acid with an amino group that may or may not be substituted
The condensation product of carboxylic acid and ammonia or an amine
Given the suffix -amide
The alkyl group on a substituted amide are written at the beginning of the name with the prefix N-
Cyclic amides = lactams; named with Greek letter of the carbon forming the bond with the N
Structural Isomers
Share only a molecular formula
Have different physical and chemical properties
Stereoisomers
Compounds with atoms connected in the same order but differing in 3D orientation
Chiral Center
Four different groups attached to a central carbon
2n Rule
n = # of chiral centers
# of stereoisomers = 2n
Conformational Isomers
Differ by rotation around a single (σ) bond
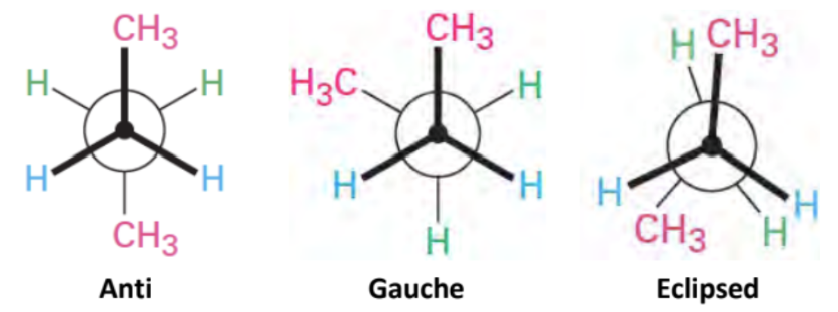
Cyclohexane Substitutients:
Equitorial
In the plane of the molecule
Cyclohexane Substitutients:
Axial
Sticking up/down from the molecule’s plane
Enatiomers
Nonsuperimposable mirror images
Opposite stereochemistry at every chiral carbon
Same chemical and physical properties, except for rotation of plane polarized light
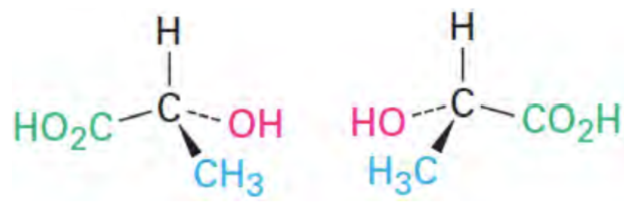
Optical Activity
The ability of a molecule to rotate plane-polarized light
d- or (+) = RIGHT
l- or (-) = LEFT
Racemis Mixture
50:50 mixture of two enantiomers
Not optically active because the rotations cancel out
Meso Compounds
Have an internal plane of symmetry, will also be optically inactive because the two sides of the molecule cancel each other out
Diastereomers
Stereoisomers that are NOT mirror image
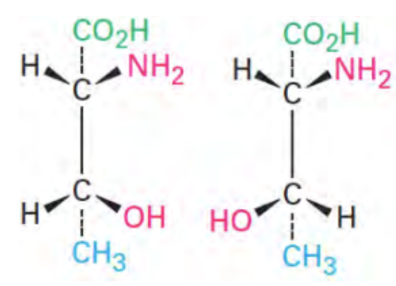
Cis-Trans
A subtype of diastereomers
Differ at some, but not all chiral centers
Different chemical and physical properties
Relative Configuration
Gives the stereochemistry of a compound in comparison to another compound
Ex. D and L
Absolute Configuration
Gives the stereochemistry of a compound without having to compare to other compounds
Ex. S and R
Cahn-Ingold-Prelog Priority Rules
Priority is given by looking at atoms connected to the chiral carbon or double-bonded carbons
Whichever has the highest atomic # gets highest priority
(Z) for Alkenes
Highest priority on same side
(E) for Alkenes
Highest priority on opposite sides
(R) and (S) for Stereocenters
A stereocenter’s configuration is determined by putting the lowest priority group in the back and drawing a circle from group 1-2-3
(R): Clockwise
(S): Counterclockwise
Fischer Projection
Vertical lines go to back of page (dashes); horizontal lines come out of the page (wedges)
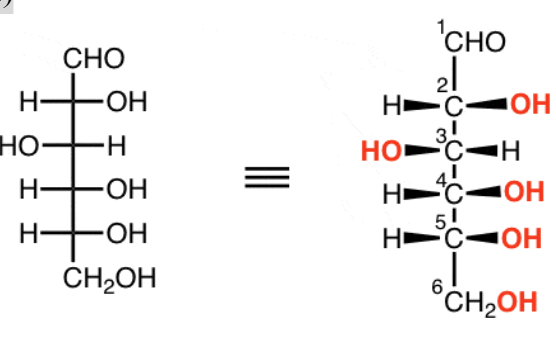
Alternating Fischer Projection
Switching 1 pair of substituents inverts the stereochemistry
Switching 2 pairs retains stereochemistry
Rotating entire diagram 90° inverts the stereochemistry
Rotating 180° retains stereochemistry
Do the compounds have the same molecular formula?
YES
Then they are Isomers
Do the compounds have the same molecular formula?
NO
Then they are different
Do the isomers have the same connectivity of atoms?
YES
Then they are stereoisomers
Do the isomers have the same connectivity of atoms?
NO
Then they are constitutional isomers
Does the interconversion of stereoisomers require breaking bonds?
YES
Then they are configurational isomers
Does the interconversion of stereoisomers require breaking bonds?
NO
Then they are conformers
Are the configurational isomers non-superimposable mirror images?
YES
Then they are enatiomers
Are the configurational isomers non-superimposable mirror images?
NO
Then they are diastereoisomers
Bonding Orbitals
Created by head-to-head or tail-to-tail overlap of atomic orbitals of the same sign
↓ energy ↑ stable
Antibonding Orbitals
Created by head-to-head or tail-to-tail overlap of atomic orbitals of opposite signs
↑ energy ↓ stable
Single Bonds
1 σ bond, contains 2 electrons
Double Bonds
1 σ and 1 𝜋
Pi bonds are created by sharing of electrons between two unhybridized p-orbitals that align side-by-side
Triple Bonds
1 σ + 2 𝜋
Multiple bonds are less flexible than single bonds because rotation is not permitted in the presence of a 𝜋 bond
Multiple bonds are shorts and stronger than single bonds, although individual 𝜋 are weaker than σ bonds
sp3 Hydridization
25% s character and 75% p character
Tetrahedral geometry with 109.5° bond angles
sp2 Hydridization
33% s character and 67% p character
Trigonal planar geometry with 120° bond angles
sp Hydridization
50% s character and 50% p character
Linear geometry with 180° bond angles
Resonance
Describes the delocalization of electrons in molecules that have conjugated bonds
Conjugation
Occurs when single and multiple bonds alternate, creating a system of unhybridized p orbitals down the backbone of the molecule through which 𝜋 electrons can delocalize
Lewis Acid
e- acceptor. Has vacant orbitals or + polarized atoms
Lewis Base
e- donor. Has a lone pair of e-, are often anions
Bronsted-Lowry Acid
Proton donor
Bronsted-Lowry Base
Proton acceptor
Amphoteric Molecules
Can act as either acids or bases, depending on reaction conditions
Ka
Acid dissociation constant. A measure of acidity
It is the equilibrium constant corresponding to the dissociation of an acid, HA, into a proton and its conjugate base
pKa
An indicator of acid strength
pKa decreases down the PT and increases with EN
pKa = -log(Ka)
⍺-carbon
A carbon adjacent to a carbonyl
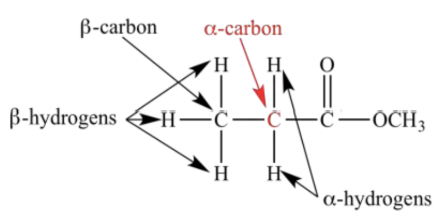
⍺-hydrogen
Hydrogen connected to an ⍺-carbon
Relatively acidic and can be removed by a strong base
The e-withdrawing O of the carbonyl weakens the C-H bonds on ⍺-hydrogens
The enolate resulting from deprotonation can be stabilized by resonance with the carbonyl
Nucleophiles
“Nucleus-loving”. Contain lone pairs or 𝜋 bonds
They have ↑ EN and often carry a NEG charge
Amino groups are common organic nucleophiles
Nucleophilicity
A kinetic property. The nucleophile’s strength
Factors that affect nucleophilicity: charge, EN, steric hindrance, and the solvent
Electrophilicity
“Electron-loving”. Contain a + charge or are positively polarized
More positive compounds are more electrophilic
Leaving Group
Molecular fragments that retain the electrons after heterolysis
The best LG can stabilize additional charge through resonance or induction
Weak bases make good LG
SN1 Reactions
Unimolecular nucleophilic substitution. 2 steps.
Step 1: LG leaves, forming a carbocation
Step 2: Nucleophile attacks the planar carbocation from either side, leading to a racemic mixture of products
Rate = k [substrate]
SN2 Reactions
Bimolecular nucleophilic substitution. 1 concerted step.
Nucleophile attacks at the same time as the LG leaves
Nucleophile must perform a backside attack, which leads to inversion of stereochemistry (R) and (S) is also changed if the nucleophile and LG have the same priority level
SN2 prefers less-substituted carbons because steric hindrance inhibits the nucleophile from accessing the electrophilic substrate carbon
Rate = k [nucleophile] [substrate]
Oxidation Number
Charge an atom would have if all its bonds were completely ionic
Oxidation
Raises oxidation state. Assisted by oxidizing agents
Aldehydes and ketones are commonly produced by oxidation of primary and secondary alcohols, respectively
Oxidizing Agent
Accepts electrons and is reduced in the process
Reduction
Lowers oxidation state. Assisted by reducing agents
Reducing Agent
Donates electrons and is oxidized in the process
Chemoselectivity
Both the nucleophile-electrophile and REDOX reactions tend to act at the highest-priority (most oxidized) functional group
One can make use of steric hindrance properties to selectively target functional groups that might not primarily react, or to protect functional groups
Polar Protic Solvents
Can Dontate H+
Favor SN1 and E1
Acetic Acid
H2O
ROH
NH3
Polar Aprotic Solvents
Can’t Donate H+
Favor SN2 and E2
DNF
DMSO
Acetone
Ethyl Acetate
Methyl Substrate in Polar Protic Solvent, Polar Aprotic Solvent, Strong Small Base, and Strong Bulky Base will Undergo ___ Reaction(s)
SN2 for ALL of the above
Methyl is too unstable to have carbocation from SN1
Primary Substrate in Polar Protic Solvent, Polar Aprotic Solvent, Strong Small Base, and Strong Bulky Base will Undergo ___ Reaction(s)
SN2: Polar Protic, Polar Aprotic, and Strong Small Base
Primary Structures are too unstable to have carbocation from SN1
E2: Strong Bulky Base
Carbon will not want to replaced LG with a large bulky Nu so it prefers the double bond
Secondary Substrate in Polar Protic Solvent, Polar Aprotic Solvent, Strong Small Base, and Strong Bulky Base will Undergo ___ Reaction(s)
SN1/E1: Polar Protic Solvent
Substrate is positively charged
Stabilized by the solvent so that’s why it is willing to do 2 steps instead of 1
SN2: Polar Aprotic Solvent
O2 won’t be able to stabilize as well so it doesn’t want to be a carbocation (1 step is better)
E2: Strong Small Base and Strong Bulky Base
Carbon will not want to replaced LG with a large bulky Nu so it prefers the double bond
Too many Cs attached for it to be SN2 and bases will just help replace LG with double bond
Tertiary Substrate in Polar Protic Solvent, Polar Aprotic Solvent, Strong Small Base, and Strong Bulky Base will Undergo ___ Reaction(s)
SN1/E1: Polar Protic and Polar Aprotic Solvents
Are able to stabilize carbocations so SN1 and E1 are okay
E2: Strong Small Base and Strong Bulky Base
Carbon will not want to replaced LG with a large bulky Nu so it prefers the double bond
Too many Cs attached for it to be SN2 and bases will just help replace LG with double bond
Substitution Reactions
Nu reacting
Nu attacking molecules and replacing LG
Pay attention to which solution it is taking place in
SN1: 2 steps, LG leaves THEN Nu attacks
SN2: 1 step, LG leaves AND Nu attacks in same step
Elimination Reactions
In a basic solution (base)
Get rid of LG and replace with double bond
E1: 2 steps, LG leaves THEN solution replaces it with double bond
E2: 1 step, LG leaves AND double bond is formed in same step