EXAM #6 ORGO
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
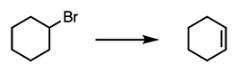
The below reaction is an example of a(n) _________ reaction.
A. Addition
B. Substitution
C. Elimination
D. Redox
E. Combustion
C
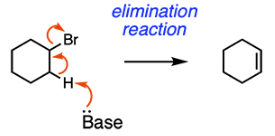
Elimination results in the formation of a carbon-carbon pi bond due to the removal of a hydrogen atom and loss of a leaving group. In the mechanism below, the base grabs a hydrogen atom. The electrons of the carbon-hydrogen sigma bond are pushed to form a carbon-carbon pi bond. Finally, the bromine leaving group leaves. This reaction, E2, follows a concerted mechanism. The reaction can also proceed E1 with a carbocation intermediate.
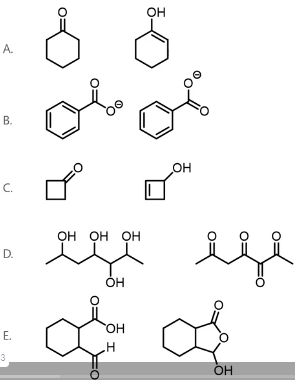
Which pair of structures represents tautomers?
Keto-enol tautomerism arises from the movement of pi electrons and protons. It is defined as the equilibrium that occurs between the keto form (ketone or aldehyde) and the enol form (hydroxyl bonded to alkene carbon), as shown below. The pair of molecules in Option A are tautomers.
Therefore, Option A is the correct answer.

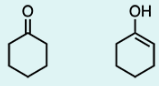
Which of the following compounds is the most stable form of C5H8?
A. HCCCH2CH2CH3
B. CH2CCHCH2CH3
C. CH2CHCHCHCH3
D. CH2CHCH2CHCH2
E. CH3CHCCHCH3
C
A primary concept to consider is resonance when analyzing the stability of molecules. Resonance is possible when atoms can accommodate the delocalization of pi electrons. Each resonance structure must maintain the same skeleton (no movement of atoms), be a valid Lewis structure, and have the same net charge. The resonance structures for an acetate ion (CH3COO) demonstrate that there are two possible forms of the ion, which indicates that this molecule is highly stable.


Before analyzing the option choices for resonance, convert each of the choices to its structural formula. Within the option choices, Option C contains two double bonds in a conjugated form – i.e. alternating double bonds. Since they are alternating, they contribute to resonance and thus make the molecule more stable. To elaborate, the overlapping p orbitals on adjacent atoms allow the electrons to be delocalized over the four or more atoms.
Which of the following is true regarding the initiation step of free radical reactions?
A. It has the lowest activation energy and will be the rate-determining step
B. It has the lowest activation energy and will not be the rate-determining step
C. It has the highest activation energy and will not be the rate-determining step
D. It has the highest activation energy and will be the rate-determining step
E. The activation energy of the initiation step is equal to the activation energy for the termination step
D
There are three steps in a free radical reaction: initiation, propagation, and termination. Initiation consists of one stable molecule splitting into two radicals (homolytic cleavage). Propagation consists of one radical reacting with a stable molecule to propagate the production of another radical. Termination consists of two radicals coming together to form a stable molecule.
Free radicals are unstable, which means their formation is energetically unfavorable. As a result, lots of energy is required to generate them. In a free radical reaction, the formation of free radicals (initiation step) has the highest activation energy and is the rate-determining step. On the other hand, in the termination step, radicals react to form stable molecules, making the reaction energetically favorable.
How many degrees of unsaturation are there in C6H7N?
A. 1
B. 2
C. 3
D. 4
E. 5
D
To calculate the degrees of unsaturation for a compound, use the following formula:
Degrees of Unsaturation = (2C + 2 + N – X – H) / 2
C = # of carbon atoms
N = # of nitrogen atoms
X = # of halogen atoms
H = # of hydrogen atoms
If we substitute 6 for C, 7 for H, and 1 for N we get: (2(6) + 2 + 1 – 0 – 7) / 2 = 4.
Using the degrees of unsaturation formula, we can conclude that the molecule has four degrees of unsaturation.
Aniline (shown below) is an example of a molecule with the molecular formula C6H7N. The molecule has 1 ring and three double bond — so it has 4 degrees of unsaturation.
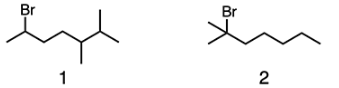
Which compound is more likely to undergo elimination in the presence of methoxide?
A. Compound 1
B. Compound 2
C. Both are equally likely to undergo elimination
D. Compound 1 due to a decreased change for substitution
E. Compound 2 due to hydrogen bonding
B
Methoxide is both a strong base and a strong nucleophile. It cannot perform nucleophilic substitution on 2-bromo-2-methylheptane (compound 2) because the alkyl halide is tertiary, and thus is very sterically hindered. Due to steric hindrance, methoxide will instead act as a base and perform elimination on compound 2. On the other hand, compound 1 is a secondary alkyl halide which is less sterically hindered than compound 2. Thus, methoxide can perform SN2 or E2 on compound 1.
How many 1H NMR signals would you expect for the following compound?
A. 1
B. 2
C. 3
D. 4
E. 5
C
The number of 1H NMR signals is equal to the number of non-equivalent protons. Protons are equivalent when they exist in the same chemical environment. If we draw a plane of symmetry through the compound, we see that it has 3 non-equivalent protons, which results in 3 distinct signals, as shown below.

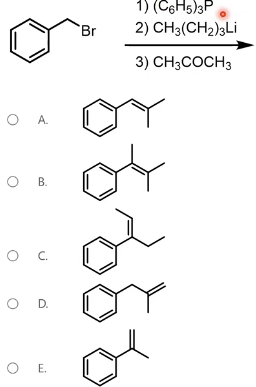
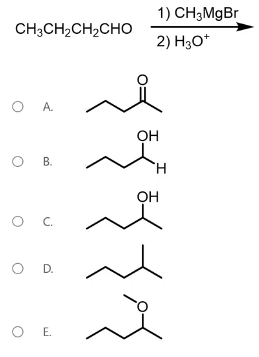
Identify the product of the following reaction sequence.
This is a Wittig reaction. Treatment of benzyl bromide with triphenylphosphine and then a strong base like n-butyllithium will prepare a Wittig reagent. The Wittig reaction will convert a ketone to an alkene. When the Wittig reagent is reacted with acetone, we get the alkene in Option A.

The carbons from the Wittig reagent and from the ketone are conserved in the final alkene product.

Note that triphenylphosphine is often abbreviated to PPh3. A phenyl (Ph) group can also appear as its formula, -C6H5. Additionally, n-butyllithium is often abbreviated to n-BuLi or BuLi. The butyl (Bu) group can also appear as its formula CH3(CH2)3-. This question demonstrates the importance of recognizing and applying molecular and structural formulas on the test. This question can be rewritten in the following format, which may be more familiar.

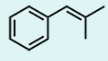
What is the product of the following reaction?
C
Recall that carbon is more electronegative than magnesium, so electron density in methyl magnesium bromide (Grignard reagent) is mostly on the methyl group. The carbon on this methyl group, which is very electron-rich, functions as a nucleophile and attacks the carbonyl carbon of the reacting aldehyde. The carbonyl carbon is electrophilic (electron-deficient) because it is double bonded to oxygen, a highly electronegative atom. When the Grignard reagent attacks the aldehyde, a pair of electrons from the pi bond are kicked up onto the oxygen to form an alkoxide ion. The alkoxide is subsequently protonated during the acidic workup to form the final product, as shown below.
Which compound will produce a strong peak at approximately 1700 cm-1on an IR spectrum?
A. CH3CH2CH2OH
B. CH3CH2CH2NH2
C. CH3CN
D. CH2CH2
E. CH3COCH3
E
Infrared (IR) spectroscopy gives us information about which functional groups are present in a molecule. Certain IR absorptions are important because they confirm the presence of a particular functional group. Below are some of the key IR signals you should know for the DAT:
A strong signal at 1700 cm-1 corresponds to a carbonyl stretch, which means the compound must contain a carbonyl group. The only carbonyl-containing compound is compound E.


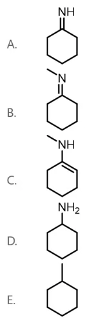
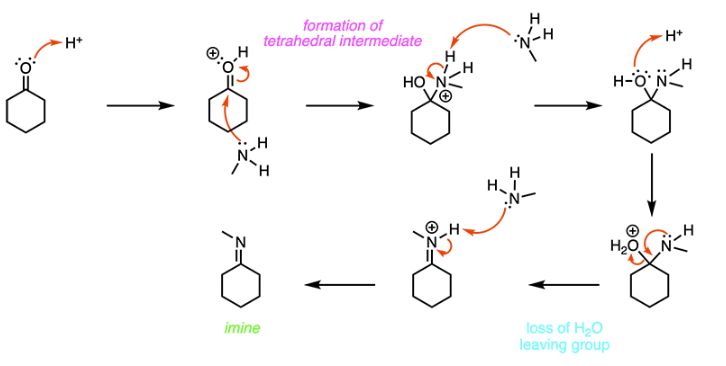
Methylamine is reacted with cyclohexanone in the presence of catalytic H2SO4. What is the final product of the reaction?
B
When a ketone is reacted with a primary amine in the presence of a catalytic acid (H2SO4), an imine is formed. In the first step of the mechanism, the carbonyl oxygen is protonated, which causes the carbonyl carbon to become more electrophilic. The amine nitrogen attacks the carbonyl carbon, and the pi electrons in the C-O double bond are pushed up onto the oxygen atom. To get rid of nitrogen’s positive charge, a proton is transferred from the amine nitrogen to the OH group. Next, a lone pair on the nitrogen moves down to form a C-N double bond, and a water molecule is kicked off. Finally, an imine is formed when the nitrogen is deprotonated, as shown below.
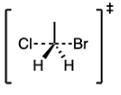
What are the partial charges on chlorine and bromine in the following pentavalent transition state?
A. Chlorine: δ+ ; Bromine: δ+
B. Chlorine: δ- ; Bromine: δ+
C. Chlorine: δ- ; Bromine: δ-
D. Chlorine: δ+ ; Bromine: δ=
E. There should be no partial charges
C
The pentavalent transition state is the highest energy configuration of atoms during an SN2 reaction. It consists of the electrophile, nucleophile (Nu), and leaving group (LG), as shown below:
IMAGE
The dashed lines represent partial bonds (bonds that are forming/breaking), and the delta +/- signs represent partial charges. There is a partial positive charge on the carbon atom because it is an electrophile, which, by definition, is electron-poor. On the other hand, the nucleophile has a partial negative charge because it is, by definition, electron-rich. The leaving group also has a partial negative charge because when it fully detaches from the molecule, it will be a negatively charged species.
Chlorine could be a leaving group or nucleophile, and the same can be said about bromine. However, regardless of their respective roles in the reaction, they both have partial negative charges.

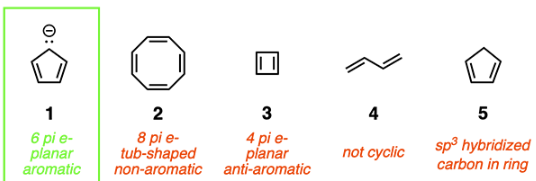
Which of the following compounds is aromatic?
A. 1
B. 2
C. 3
D. 4
E. 5
A
The rules for aromaticity are as follows:
1. The compound must be cyclic.
2. The ring must be fully conjugated and planar (none of the atoms can be sp3 hybridized).
3. The system must satisfy Huckel’s rule (# of pi electrons = 4n + 2, where n is any integer).
If a compound satisfies all the above rules, it is aromatic. If a compound satisfies all the rules except Huckel’s rule, it is anti-aromatic. If a compound fails to satisfy the first two rules, it is non-aromatic. Compound 1 is the only compound that satisfies all three rules, as shown below. Note that the negatively charged carbon may seem sp3 hybridized, but it is actually sp2 hybridized because the lone pair is delocalized around the ring.
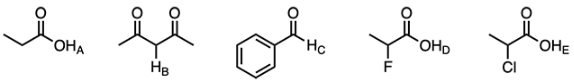
Identify the least acidic hydrogen.
A.
B.
C.
D.
E.
C
Acid strength is determined by conjugate base stability. To solve this problem, use the mnemonic CARDIO (charge, atom, resonance delocalization, induction, orbital). Keep in mind, we are looking for the least acidic hydrogen, which will be the hydrogen atom that results in the least stable conjugate base when removed.
The ‘C’ in CARDIO stands for Charge, however, all of the answer choices are neutral molecules. Therefore, this characteristic cannot be used to eliminate options.
The ‘A’ in CARDIO stands for Atom. A bigger or more electronegative atom will increase acidity because it weakens the bond to the acidic proton. Options A, D, and E are each a hydrogen atom bonded to oxygen. Options B and C are both a hydrogen atom bonded to carbon. Oxygen is more electronegative so it will better stabilize a negative charge. Therefore, options A, D, and E can be eliminated because the hydrogen atoms bonded to oxygen are more acidic than those bonded to carbon.
The ‘RD’ in CARDIO stands for Resonance Delocalization. If the conjugate base of an acid is capable of resonance, the conjugate base becomes more stable, which in turn increases acidity. When the hydrogen atom in option B is removed, it results in a resonance-stabilized conjugate base (increasing the acidity). In contrast, when the hydrogen atom in option C is removed it results in a negative charge on a carbonyl carbon.
In summary, Proton C is the least acidic hydrogen — the hydrogen of an aldehyde has a very high pKa and will not be deprotonated. The hydrogen of the aldehyde is the least acidic due to the extreme instability of the conjugate base that would result upon deprotonation. In contrast, Proton D is the most acidic because carboxylic acids are more acidic than ketones and aldehydes. Additionally, proton D is near fluorine, which is a very electronegative atom that stabilizes the conjugate base through induction.
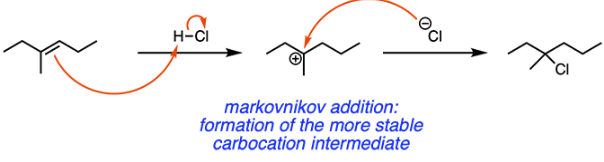
An unknown compound is reacted with HCl. The final product of the reaction is identified to be 3-chloro-3-methylhexane. Which of the following compounds could be the unknown?
A
The reaction that occurs here is a Markovnikov halogenation. If we think retrosynthetically, Option A is the only compound that will react with HCl to form the desired product, as shown below.
A separatory funnel contains water and dichloromethane. Dichloromethane is denser than water. If NH3 were added into this funnel, where would it be found?
A. The top layer (organic)
B. The top layer (aqueous)
C. The middle at the interface between the organic and aqueous layers
D. The bottom layer (organic)
E. The bottom layer (aqueous)
B
In chemistry labs, the separatory funnel allows chemists to separate immiscible liquids. We are told that dichloromethane is denser than water, so the organic DCM layer will be found below the aqueous water layer. NH3and water are both capable of donating and accepting hydrogen bonds, and thus ammonia will be located in the aqueous (top) layer. On the other hand, dichloromethane is incapable of hydrogen bonding, so it will be confined to the organic layer. Be careful — usually, the organic layer is above the aqueous layer, however, DCM is denser than water (the density was stated in the question). Ammonia will be found at the top of the aqueous layer.
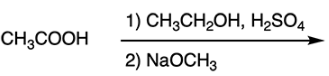
What is the product of the reaction?
A. CH3COOCH2CH3
B. CH3COOCH3
C. CH3CH2COOCH3
D. CH3CH2COOH
E. CH3COCH3
B
When the carboxylic acid is reacted with ethanol (CH3CH2OH) and sulfuric acid, an ester is formed through Fischer esterification. Transesterification will occur when the resulting ester is reacted with methoxide, as shown below.
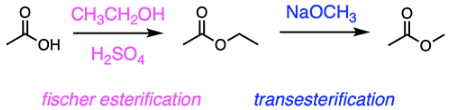
Which of the following IUPAC names is correct?
A. 2-ethylpentane
B. 2-cyclbutylpropane
C. 2,2-diethylbutane
D. 1-ethyl-5-methylcyclohexane
E. 4-ethyl-3,3-dimethylhexane
E
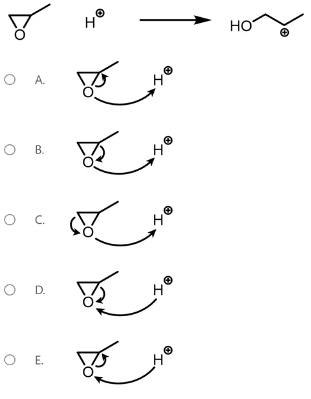
Given the following reactant and product, predict the arrow-pushing mechanism.
B
Arrow-pushing mechanisms are a way to depict electron flow as old bonds are broken and new bonds are formed during the progression of a reaction.
The arrows show the movement of electrons. A double-headed arrow indicates the movement of an electron pair while a single-headed arrow indicates the movement of a single electron. This question is an epoxide ring opening in acidic conditions.

Arrow 2 indicates the lone pair on oxygen moving to form a new bond between oxygen and hydrogen, protonating the oxygen (yellow). Arrow 1 indicates the electrons from the C-O bond moving to form a lone pair on oxygen (purple).
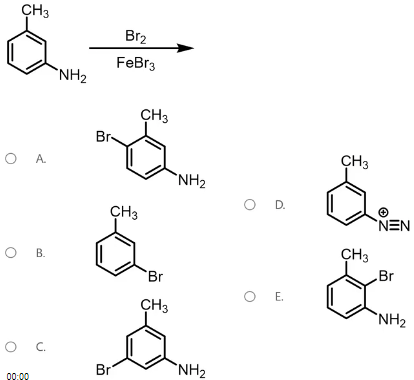
Which is the product of the foVllowing reaction?
A
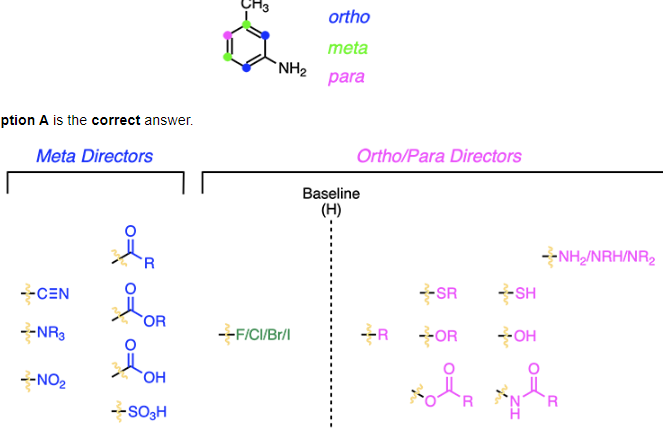
The reaction being described is an electrophilic aromatic substitution reaction. These reactions rely on being able to correctly identify what substituent the reagents add to the ring and in what location. The reagents Br2 and FeBr3 add a Br group to the aromatic ring. The location of this group is based on what is already present on the ring, which is an alkyl group and amine. Substituents that are electron donating are designated as activating groups and will direct incoming substituents into the ortho/para position. Substituents that are electron withdrawing are designated as deactivating groups and will direct incoming substituents into the meta position. Both the alkyl and amine group are activating and will direct the incoming substituent into the ortho and para position, however, the amine group is a stronger activator. This is because the amine’s lone pair of electrons, it can push electrons into the ring making the ortho and para positions more reactive. Due to less steric hindrance at the para position, that position is more favored.
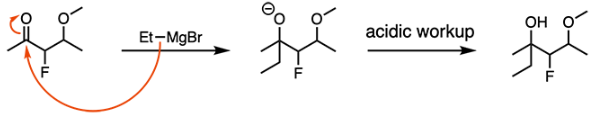
Which of the following carbons will undergo nucleophilic attack in the presence of bromoethane that has been treated with magnesium metal?
A. 1
B. 2
C. 3
D. 4
E. 5
B
Bromoethane treated with magnesium forms a Grignard reagent. Grignard reagents are strong nucleophiles that attack carbonyl carbons. Reaction of a ketone with a Grignard reagent generates a tertiary alcohol after acidic workup, as shown below.
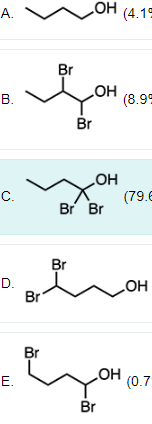
Which of the following compounds is the strongest acid?
C
The acidity of the compounds can be assessed by the stability of the respective conjugate bases. To compare the stability of conjugate bases, use the acronym CARDIO (charge, atom, resonance delocalization, induction, orbital hybridization). In the case of the given compounds, charge, atom, and resonance delocalization are not applicable as the acidic proton is the same for all compounds.
Next, compare inductive effects. Bromine helps to disperse the negative charge of the conjugate base through induction, as bromine is highly electronegative. An increased amount of bromine atoms leads to a more stable conjugate base and consequently a more acidic compound. Proximity to bromine also plays a role as this is a key factor within the inductive event. To elaborate, the closer the electronegative atoms are to the acidic proton, the stronger the inductive effect, and thus the stronger the stability of the conjugate base.
Option C has 2 bromine atoms residing on the alpha carbon, indicating the closest proximity to the acidic proton. This compound has the strongest inductive effect, making it the most stable.
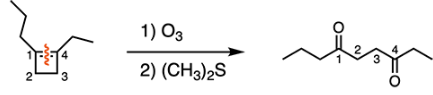
A cyclic alkene is treated with ozone followed by (CH3)2S to generate the compound below. How many carbons did the ring originally consist of?
A. 3
B. 4
C. 5
D. 6
E. 7
B
Treatment of an alkene with ozone followed by (CH3)2S will accomplish ozonolysis with a reductive workup. This reaction will cleave the carbon-carbon double bond to form two carbonyl groups. In order to figure out how many carbons the ring consisted of, count the number of carbons from one carbonyl carbon to the other:
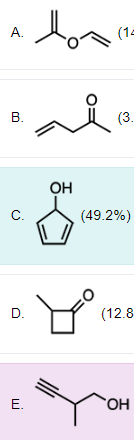
Each of the following compounds is a constitutional isomer of cyclopentanone EXCEPT one. Which is the EXCEPTION?
C
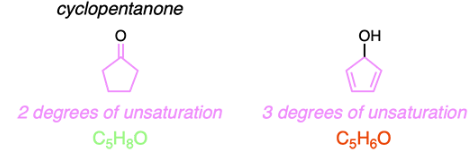
Structural (constitutional) isomers are compounds with the same molecular formula but different connectivity of atoms. To have the same molecular formula, isomers must contain the same number of degrees of unsaturation as each degree of unsaturation indicates 2 less hydrogens are present compared to the number of hydrogen atoms if the hydrocarbon were fully saturated. A pi bond or a ring will cause one degree of unsaturation.
The molecular formula of cyclopentanone is C5H8O and it contains two degrees of unsaturation in the form of two pi bonds. The only option that does not have the same molecular formula as cyclopentanone is Option C as it contains 3 degrees of unsaturation in the form of two pi bonds and one ring.
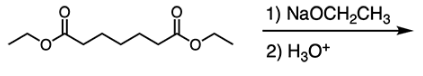
What is the name of the reaction that occurs?
A. Self aldol condensation
B. Crossed aldol condensation
C. Self Claisen condensation
D. Crossed Claisen condensation
E. Dieckmann condensation
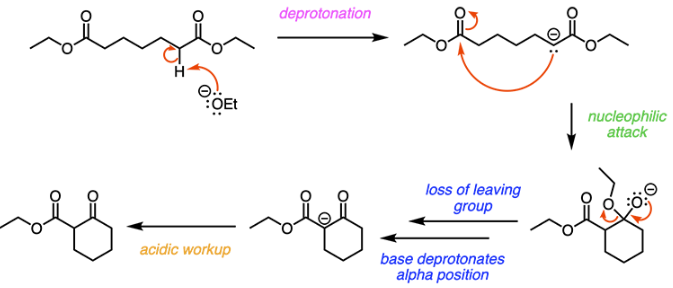
E
The Dieckmann Condensation is an intramolecular Claisen condensation. Claisen condensation reactions occur between two esters. Because Dieckmann condensation is an intramolecular Claisen condensation, a cyclic product is formed, as shown below:
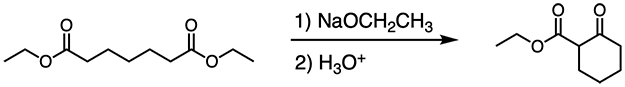
In the first step, ethoxide deprotonates the alpha carbon of an ester. The resulting enolate attacks the carbonyl carbon of the other ester. This forms a cyclic, tetrahedral intermediate. The intermediate then collapses and a ketone is formed when the ethoxy group on the ester is knocked off as a leaving group, as shown below.
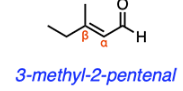
An aldol condensation is performed in an experiment. What is a possible name for a product?
A. 2-methyl-4-pentenal
B. 3-ethyl-3-hexenal
C. 3-hydroxypropanal
D. 2-hydroxybutanal
E. 3-methyl-2-pentenal
E
Aldol condensation transforms a carbonyl (aldehyde/ketone) into an α, β unsaturated carbonyl. The phrase "α, β unsaturation" implies that the alkene occurs between the alpha carbon (one carbon away) and the beta carbon (two carbons away) adjacent to the carbonyl group. 3-methyl-2-pentenal (shown below) is the only α, β unsaturated carbonyl listed in the answer options. We can determine this by analyzing the molecule's name. The "-al" suffix in 3-methyl-2-pentenal suggests that the molecule contains an aldehyde, and aldehydes are always on carbon 1 of the parent chain. The "2" in front of pentenal suggests that the alkene starts on carbon 2, the alpha carbon in this case, and ends on carbon 3, the beta carbon in this case.
Though options A and B contain aldehydes and alkenes, the alkene in each molecule is not between the alpha and beta carbons. Options C and D are also incorrect because while they both contain aldehydes (and alcohols), neither of them contains an alkene.
Which of the following compounds will produce three 13C NMR signal?
B
The number of signals on a 13C NMR spectrum will be equal to the number of non-equivalent carbon atoms. This refers to the carbons that are in chemically different environments. Option B has a plane of symmetry that creates only three distinct chemical environments.

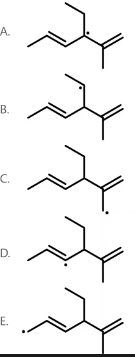
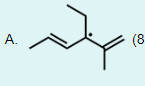
Which of these radical structures is the most stable?
A
A free radical is an atom or a molecule with an unpaired electron. Free radicals are stabilized by neighboring alkyl groups because they donate electron density to the carbon bearing the unpaired electron. In addition to neighboring alkyl groups, resonance stabilizes a free radical because it causes the unpaired electron to be delocalized over a larger area. Below are various radicals shown in order of increasing stability from left to right:

The molecule in Option A is most stable because the radical is located on a tertiary carbon, and due to the presence of neighboring pi bonds, it is also stabilized by resonance.
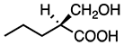
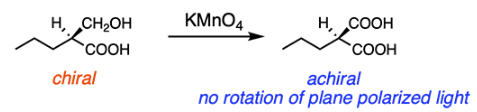
Which reagent would transform the following molecule to a molecule that does not rotate plane-polarized light?
A. CH3OH
B. NaBH4, EtOH
C. KMnO4
D. FeBr3
E. H2O
C
Reaction with the strong oxidating agent KMnO4 will transform the hydroxyl group on the molecule into a carboxylic acid (COOH). This will make the molecule achiral because the chiral carbon will no longer be attached to four different groups. Achiral molecules do not rotate plane-polarized light, as shown below.
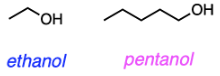
Which of the following dissolves better in water: CH3CH2OH or CH3(CH2)4OH?
A. Ethanol due to a shorter hydrocarbon chain
B. Ethanol due to stronger Van der Waals interactions with water
C. Pentanol due to a longer hydrocarbon chain
D. Pentanol due to stronger hydrogen bonding with water
E. Must be determined experimentally
A
Ethanol and pentanol (shown below) are both primary alcohols that form hydrogen bonds with water. However, the hydrocarbon chains of the two alcohols differ in size, and this directly impacts their solubility in water. A hydrocarbon chain is nonpolar, so it will not interact with water and aggregates with other hydrophobic groups. The larger hydrocarbon chain on pentanol disrupts hydrogen bonding between water molecules and the alcohol, which is energetically unfavorable. Thus, ethanol with its shorter hydrocarbon chain will dissolve better in water.