A-Level Chemistry - Topic 2 - Bonding and Structure
0.0(0)
0.0(0)
Card Sorting
1/137
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
138 Terms
1
New cards
What is an ionic bond?
the strong electrostatic attraction between two oppositely charged ions
2
New cards
What are the two things affecting the strength of an ionic bond?
ionic charged
ionic radii
ionic radii
3
New cards
How does the melting point of ions with a charge of 1 compared to ions with a charge of 2?
ions with a charge of 2 have a higher melting point
4
New cards
What is charge density?
the ratio of the charge of an ion to its volume
5
New cards
How does ionic radii affect the melting point of an ionic compound?
smaller ions = pack closer together than larger ions
smaller + closely packed ions have stronger ionic bonding
smaller + closely packed ions have stronger ionic bonding
6
New cards
What does the size of an ion depend on?
its electron shells
and atomic number
and atomic number
7
New cards
How does the ionic radius change as you go down a group?
it increases
8
New cards
Why does the ionic radius increase as you go down a group?
the ionic radius increases as the atomic number increases
(due to the extra added electron shells)
(due to the extra added electron shells)
9
New cards
What are isoelectronic ions?
ions of different atoms with the same number of electrons
10
New cards
How does the ionic radius of a set of isoelectronic ions change as the atomic number increases?
the ionic radius of a set of isoelectronic ions decreases
11
New cards
What are ionic crystals?
giant lattices of ions
12
New cards
Why are ionic lattices called "giant"?
because it's made up of the same basic unit repeated over and over again
13
New cards
What is the shape of sodium chloride's lattice?
cube shaped
14
New cards
What does the theory of ionic bonding fit?
the evidence from physical properties
15
New cards
What are the main physical properties of ionic compounds?
high melting points
soluble in water (but not in non-polar solvents)
don't conduct electricity when solid but do when molton/dissolved/aqueous
can't be shaped due to repulsion = shatter
soluble in water (but not in non-polar solvents)
don't conduct electricity when solid but do when molton/dissolved/aqueous
can't be shaped due to repulsion = shatter
16
New cards
What are ionic compounds not soluble in?
non-polar solvents
17
New cards
What happens when you electrolyse a green solution of copper(II) chromate(VI)?
filter paper turns blue at the cathode
turns yellow at the anode
turns yellow at the anode
18
New cards
What colour are copper(II) ions in solution?
blue
19
New cards
What colour are chromate(VI) ions in solition?
yellow
20
New cards
Why is Copper(II) chromate(VI) green?
contains both copper(II) ions - blue - and chromate(VI) ions - yellow
21
New cards
What else is present (in terms of attraction/repulsion) when covalent bonding occurs?
positive nuclei attracted to the area of electron density between the 2 nuclei
but also a repulsion (2 positively charged nuclei repel each other, same with electrons)
to maintain a covalent bond = have to be a balance between these forces
but also a repulsion (2 positively charged nuclei repel each other, same with electrons)
to maintain a covalent bond = have to be a balance between these forces
22
New cards
What is the "bond length"?
the distance between 2 nuclei is the distance where the attractive and repulsive forces balance each other
= the bond length
= the bond length
23
New cards
How does the bond enthalpy change in terms of electron density?
higher the electron density between the nuclei (the more electrons in the bond)
= the stronger the attraction between the atoms
= the higher the bond enthalpy
= the shorter the length of the bond
= the stronger the attraction between the atoms
= the higher the bond enthalpy
= the shorter the length of the bond
24
New cards
What is bond enthalpy?
energy required to break a bond
25
New cards
How does a C=C bond compare to a C-C bond?
C=C = greater bond enthalpy and shorter than a C-C bond
26
New cards
What is a dative covalent bond also called?
a coordinate bond
27
New cards
What is a dative covalent bond?
where an atom donates both electrons to a bond
28
New cards
How is NH4+ formed?
by a dative covalent bond
it forms when the nitrogen atom in an ammonia molecule donates a pair of electrons to a proton (H+)
it forms when the nitrogen atom in an ammonia molecule donates a pair of electrons to a proton (H+)
29
New cards
How is dative covalent bonding represented?
as an arrow pointing AWAY from the donor atom
30
New cards
What is an example of a stable covalent compound where the central atom doesn't have a full outer shell?
AlCl3
since Al only has 6 electrons in its outer shell
since Al only has 6 electrons in its outer shell

31
New cards
What do 2 AlCl3 compounds come together to form?
Al2Cl6
one Cl in each of the two AlCl3 molecules donate a lone pair to the Al on the other molecule
= form 2 dative covalent bonds, allowing Al to have a full outer shell
one Cl in each of the two AlCl3 molecules donate a lone pair to the Al on the other molecule
= form 2 dative covalent bonds, allowing Al to have a full outer shell
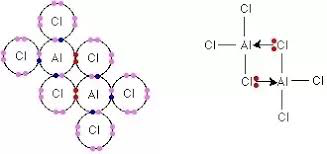
32
New cards
What does the shape of a molecule depend on?
the number of pairs of electrons in the outer shell of the central atom
33
New cards
What will electron pairs do to each other?
repel each other as much as they can
34
New cards
What also affects how much electron pair repel each other?
the type of electron pair
35
New cards
Which repels each other more: lone pairs or bonding pairs?
lone pars repel more than bonding pairs
36
New cards
Where are the greatest angles in molecules?
between lone pairs of electrons
= bond angles between bonding pairs are reduced
= bond angles between bonding pairs are reduced
37
New cards
Why are bonding pairs reduced?
since they are pushed together by lone pair repulsion
38
New cards
What is the order of repulsion in terms of electrons?
LP/LP (biggest)
LP/BP
BP/BP (smallest)
LP/BP
BP/BP (smallest)
39
New cards
What is electron repulsion theory?
electron pairs repel each = they
position themselves as far apart as possible
all bonding electron pairs repel each other
equally
lone pairs offer more repulsion than bonded
pairs
position themselves as far apart as possible
all bonding electron pairs repel each other
equally
lone pairs offer more repulsion than bonded
pairs
40
New cards
What is the bond angle for methane?
no lone pairs
all bond angles are 109.5
all bond angles are 109.5
41
New cards
What is the bond angle for ammonia?
1 lone pair of electrons
all 3 bond angles are 107
all 3 bond angles are 107
42
New cards
What is the bond angle for water?
2 lone pairs = reduce the bond angle even more
bond angle is 104.5
bond angle is 104.5
43
New cards
How are 3D molecular diagrams drawn?
solid wedges = pointing out of the page
broken lines = bonds pointing into the page
broken lines = bonds pointing into the page
44
New cards
How can you predict the shape of a molecule?
1. find the central atom
2. work out the number of electrons in the outer shell of it
3. work out how many electron are shared with the central atom
4. add up the electrons and divide by 2 (to find the number of electron pairs on the central atom)
5. compare the number of electron pairs with the number of bonds to find the number of lone pairs
6. use the number of electron pairs + lone pairs + bonding centres around the central atom to work out the shape of the molecule
2. work out the number of electrons in the outer shell of it
3. work out how many electron are shared with the central atom
4. add up the electrons and divide by 2 (to find the number of electron pairs on the central atom)
5. compare the number of electron pairs with the number of bonds to find the number of lone pairs
6. use the number of electron pairs + lone pairs + bonding centres around the central atom to work out the shape of the molecule
45
New cards
What is a "bonding centre"?
the atoms bonded to the central atom
46
New cards
What is the bonding angle and name for 2 electron pairs around a central atom?
180
linear molecules
linear molecules
47
New cards
What is the bonding angle and name for 3 electron pairs around a central atom with no lone pairs?
120
trigonal planar
trigonal planar
48
New cards
What is the bonding angle and name for 4 electron pairs around a central atom with no lone pairs?
109.5
tetrahedral
tetrahedral
49
New cards
What is the bonding angle and name for 4 electron pairs around a central atom with 1 lone pair?
107
trigonal pyramidal
trigonal pyramidal
50
New cards
What is the bonding angle and name for 4 electron pairs around a central atom with 2 lone pairs?
104.5
nonlinear/"bent"
nonlinear/"bent"
51
New cards
What is the bonding angle and name for 5 electron pairs around a central atom with no lone pairs?
120 and 90
trigonal bipyramidal
trigonal bipyramidal
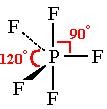
52
New cards
What is the bonding angle and name for 6 electron pairs around a central atom with 0 lone pairs?
90
octahedral
octahedral
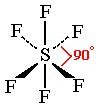
53
New cards
What is the bonding angle and name for 6 electron pairs around a central atom with 2 lone pairs?
90
square planar
square planar
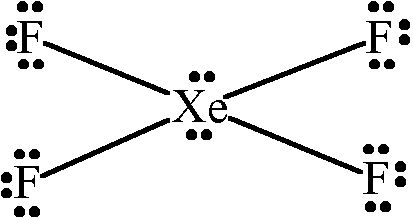
54
New cards
What is the shape of diamond (and silicon, too)?
each carbon atom bonded to 4 others
in a tetrahedral arrangement
in a tetrahedral arrangement
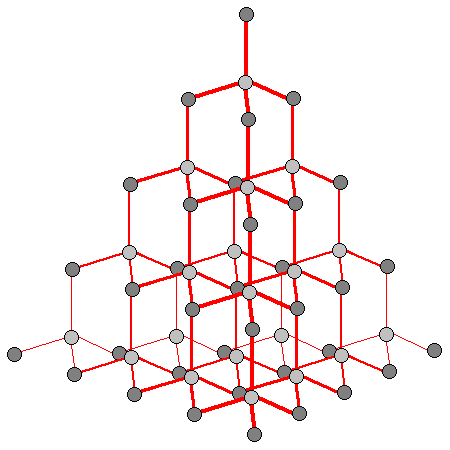
55
New cards
What is the shape of Silicon(IV) dioxide?
similar but different lattice arrangement to diamond
56
New cards
What is a tetrahedron?
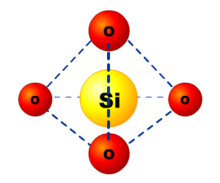
57
New cards
What do the properties of giant structures provide evidence for?
covalent bonding
58
New cards
What are the properties of giant covalent structures?
high melting points
very hard
good thermal conductors
insoluble
cant conduct electricity
very hard
good thermal conductors
insoluble
cant conduct electricity
59
New cards
Why are giant covalent structures good thermal conductors?
since vibrations travel easily through the stiff lattices
60
New cards
Why are giant covalent structures insoluble?
covalent bonds = atoms are more attracted to their neighbours in the lattice than to solvent molecules
since they're insoluble in polar solvents = displays they don't contain ions
since they're insoluble in polar solvents = displays they don't contain ions
61
New cards
What is an example of a polar solvent?
water
62
New cards
How many other carbon atoms is graphite bonded to?
3 other = delocalised electrons
63
New cards
Why do metals have high melting + boiling points?
because of the strong metallic bonding
(number of delocalised electrons, the charge, the size of the metal ion and the lattice structure affect the m.p)
(number of delocalised electrons, the charge, the size of the metal ion and the lattice structure affect the m.p)
64
New cards
Why are metals malleable + ductile?
since there's no bonds holding specific ions together
+ the layers of positive metal ions are separated by layers of electrons
= the layers of metal ions can slide over each other without disrupting the attraction between the positive ions and electrons
+ the layers of positive metal ions are separated by layers of electrons
= the layers of metal ions can slide over each other without disrupting the attraction between the positive ions and electrons
65
New cards
Why are metals good thermal conductors?
since they can pass kinetic energy to each other
66
New cards
What could impurities in metals do and why?
dramatically reduce electrical conductivity
by reducing the number of electrons that are free to move and carry charge
the electrons transfer to the impurities = forming anions
by reducing the number of electrons that are free to move and carry charge
the electrons transfer to the impurities = forming anions
67
New cards
Why are metals insoluble and what is the exception to this?
because of the strength of the metallic bonds
EXCEPTION: liquid metals
EXCEPTION: liquid metals
68
New cards
What is electronegativity?
the ability of an atom to attract the bonding electrons in a covalent bond
69
New cards
How is electronegativity measured?
pauling scale (0-4)
70
New cards
What is the most electronegative element?
flourine (4.0)
71
New cards
What are other examples of very electronegative elements?
oxygen
chlorine
nitrogen
chlorine
nitrogen
72
New cards
What are the values of the least electronegative elements?
around 0.7
73
New cards
What are the factors that contribute to having such a high electronegativity?
higher nuclear charges
smaller atomic radii
smaller atomic radii
74
New cards
How does electronegativity change across periods and down groups?
across periods: increasing electronegativity
down groups: decreasing electronegativity
down groups: decreasing electronegativity
75
New cards
Where do the bonding electrons sit within covalent bonding?
in orbitals between the two nuclei
76
New cards
What happens if both atoms have similar/identical electronegativities?
the bonding electrons with sit roughly sit midway between the 3 nuclei
= the bond will be non-polar
= the bond will be non-polar
77
New cards
What happens when the bonding atoms have very similar electronegativities?
the bonds between them are essentially non-polar
78
New cards
What happens to the bonding electrons when there's a relatively strong electronegativity?
the bonding electrons will be pulled more towards the electronegative atom
= causes the electrons to be spread unevenly
= there will be a charge across the bond
= the bond is said to be polar
EACH ATOM HAS A PARTIAL CHARGE (ONE ATOM IS SLIGHTLY POSITIVE AND THE OTHER IS SLIGHTLY NEGATIVE)
= causes the electrons to be spread unevenly
= there will be a charge across the bond
= the bond is said to be polar
EACH ATOM HAS A PARTIAL CHARGE (ONE ATOM IS SLIGHTLY POSITIVE AND THE OTHER IS SLIGHTLY NEGATIVE)
79
New cards
Within a polar bond, what does the difference in electronegativity cause?
a dipole
80
New cards
What is a dipole?
a difference in charge between the 2 atoms caused by a shift in electron density in the bond
81
New cards
What does "𝛿" mean?
slightly
82
New cards
What are the 2 extremes when talking about bonding?
purely covalent (electronegativity = 0)
= bonding electrons are arranged completely evenly within the bond
completely ionic
= bonding electrons are arranged completely evenly within the bond
completely ionic
83
New cards
Where do most compounds lie?
in the middle of the 2 bonding extremes
= often got ionic AND covalent properties
= often got ionic AND covalent properties
84
New cards
What does a higher difference in electronegativity mean?
the more ionic in character the bonding becomes
85
New cards
When are bonds polar?
if the difference in electronegativity is > 0.4
86
New cards
What does whether a molecule is polar or not depend on?
its shape
+ the polarity of its bonds
+ the polarity of its bonds
87
New cards
What does a polar molecule have?
an overall dipole
88
New cards
What is an overall dipole within a polar molecule?
a dipole caused by the presence of a permanent charge across the molecule
89
New cards
How can you tell if a molecule with several polar bonds is a polar molecule?
if the polar bonds are arranged so that they point in opposite directions
= they're point each other out
= non-polar overall
if the polar bonds all point in roughly the same direction
= then the molecule will be polar
= they're point each other out
= non-polar overall
if the polar bonds all point in roughly the same direction
= then the molecule will be polar
90
New cards
What are intermolecular forces?
forces between molecules
much weaker than covalent/ionic/metallic bonds
much weaker than covalent/ionic/metallic bonds
91
New cards
What are the three types of intermolecular bonds?
london forces (instantaneous dipole-induced dipole bonds)
permanent dipole-permanent dipole bonds
hydrogen bonding
permanent dipole-permanent dipole bonds
hydrogen bonding
92
New cards
What is the strongest type of intermolecular bond?
hydrogen bonding
93
New cards
What are london forces also called?
instantaneous dipole-induced dipole bonds
94
New cards
What do london forces cause?
all atoms and molecules to be attracted to each other
95
New cards
Why do london forces occur?
1. electrons in charge clouds are always moving really quickly so at any moment the electrons in an atom are likely to be more to one side than the other
= temporary/instantaneous dipole occurs
2. this dipole can induce another temporary dipole in the opposite direction = these 2 dipoles are attracted to each other
= DOMINO EFFECT
3. since electrons are constantly moving = dipoles are being created + destroyed all the time
= despite the dipoles keep changing, the overall effect = atoms attracted to each other
= temporary/instantaneous dipole occurs
2. this dipole can induce another temporary dipole in the opposite direction = these 2 dipoles are attracted to each other
= DOMINO EFFECT
3. since electrons are constantly moving = dipoles are being created + destroyed all the time
= despite the dipoles keep changing, the overall effect = atoms attracted to each other
96
New cards
What can london forces hold molecules together in?
a lattice
e.g. Iodine = I2 molecules held via covalent bonds = molecules then held together in a molecular lattice by weak london forces
= called the simple molecular structure
e.g. Iodine = I2 molecules held via covalent bonds = molecules then held together in a molecular lattice by weak london forces
= called the simple molecular structure
97
New cards
Why aren't all london forces the same strength?
larger molecules = larger electron clouds = stronger london forces
98
New cards
What does a molecule with a greater surface area have and why?
stronger london forces
= since they have a bigger EXPOSED electron cloud
= since they have a bigger EXPOSED electron cloud
99
New cards
What do you need to overcome when boiling something?
intermolecular forces
= so that the particles can escape from the liquid surface
= so that the particles can escape from the liquid surface
100
New cards
What do stronger london forces mean?
a higher b.p.