The History of the Atom
1/12
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What are atoms?
The tiny particles of matter (stuff that has mass) which makes up everything in the universe
What was the theory of atomic structure at the start of the 19th century?
John Dalton described atoms as solid spheres, and said that different spheres made up the different elements
What was the theory of atomic structure in 1897?
JJ Thompson concluded from his experiments that atoms weren’t solid spheres. His measurements of charge and mass showed that an atom must contain even smaller, negatively charged particles - electrons
What was the theory of atomic structure after the ‘solid sphere’ idea?
The ‘Plum pudding model’
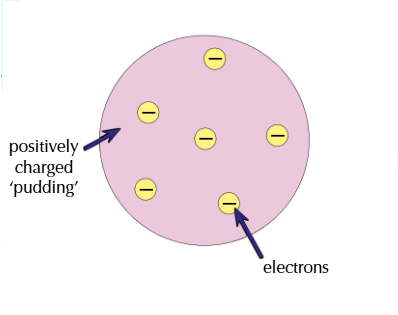
How did the plum pudding model show the atom?
As a ball of positive charge with electrons stuck in it
How did Rutherford show that the Plum Pudding Model was wrong?
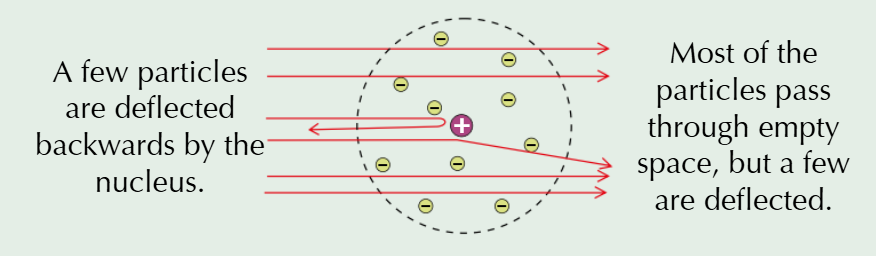
In 1909, him and his students conducted the famous gold foil experiment. They fired positively charged alpha particles at an extremely thin sheet of gold.
What did Rutherford and his students expect to happen when conducting his gold foil experiment?
They were expecting the particles to pass straight through the sheet or be slightly deflected (from the PPM) at most. This was because the positive charge of each atom was though to be very spread out through the ‘pudding’ of the atom.
What actually happened when Rutherford and his students conducted his gold foil experiment?
Whilst most of the particles did go straight through the gold sheet, some were deflected more than expected, and a small number were deflected backwards, therefore the PPM couldn’t be right
What did Rutherford come up with to explain his new evidence?
The ‘nuclear model’ of the atom. In this, there’s a tiny, positively charged nucleus at the centre, where most of the mass is concentrated. A ‘cloud’ of negative electrons surrounds this nucleus - so most of the atom is empty space. When alpha particles came near the concentrated, positive charge of the nucleus, they were deflected. If they were fired directly at the nucleus, they were deflected backwards. Otherwise, they passed through the empty space.
What did Scientists realise about ?
That electrons in a ‘cloud’ around the nucleus of an atom, as Rutherford described, would be attracted to the nucleus, causing the atom to collapse.
Who proposed the idea that electrons were contained in shells?
Niels Bohr - proposed that electrons orbit the nucleus in fixed shells and aren’t anywhere in between. Each shell is a fixed distance from the nucleus and has a fixed energy
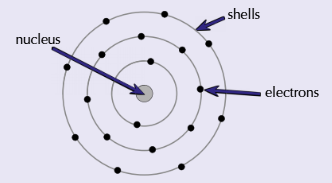
Which model is pretty close to out currently accepted version of the atom?
The Bohr model
What was Bohr’s theory of atomic structure supported by?
Many experiments and it helped to explain lots of other scientists’ observations at the time