CELL 1010 CH 3 Dr. V Tulane University
1/134
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
135 Terms
molecule vs compound
molecule = chemical particle composed of two or more atoms united by a chemical bond (O2); compound = molecules composed of two or more elements (H20)
Functional groups
chemical groups that have not fulfilled the octet rule
functional groups exhibit the same properties in all molecules they are present in
Isomers
contain same atoms but in different bonding relationships and spatial organization, affecting chemical properties
Structural Isomers
Compounds that have same molecular formula but differ in bonding order. Synonymous with constitutional isomer. Found in alkanes. Branching.
geometric isomers
Compounds that have the same molecular formula but differ in the spatial arrangements of their atoms.
Found in alkenes and arenes (presence of double bond)
a subset of stereoisomer
cis-trans isomers
have the same covalent bonds but differ in spatial arrangements
stereoisomer
Enantiomers
mirror image of another molecule. has left and right side.
a subset of Stereoisomer
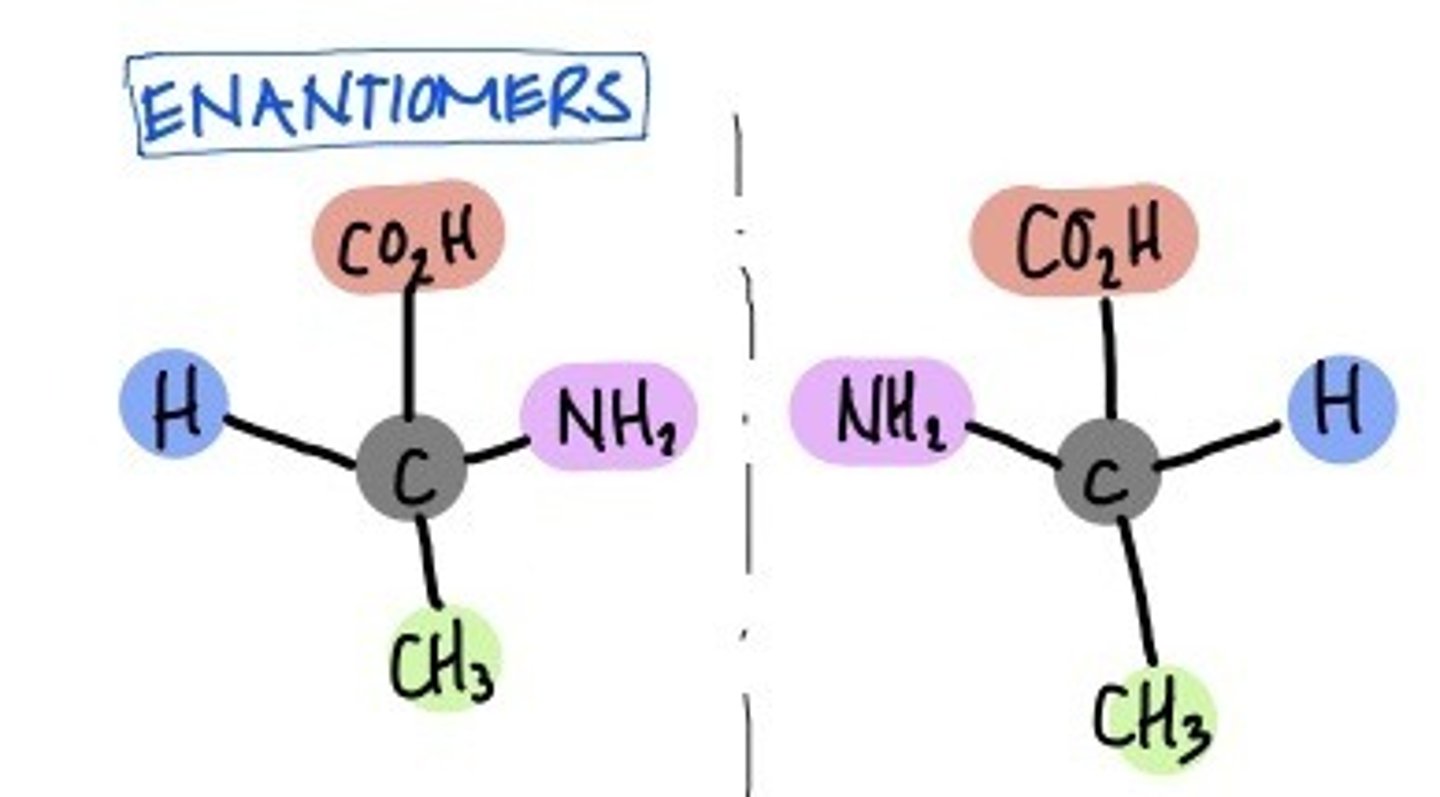
Structural Isomers vs stereoisomers
structural: different bonding
(ie. linear form of glucose, fructose)
stereo: different spatial organization
(ie. alpha & beta glucose, linear form of glucose and galactose, ring form L vs D glucose)
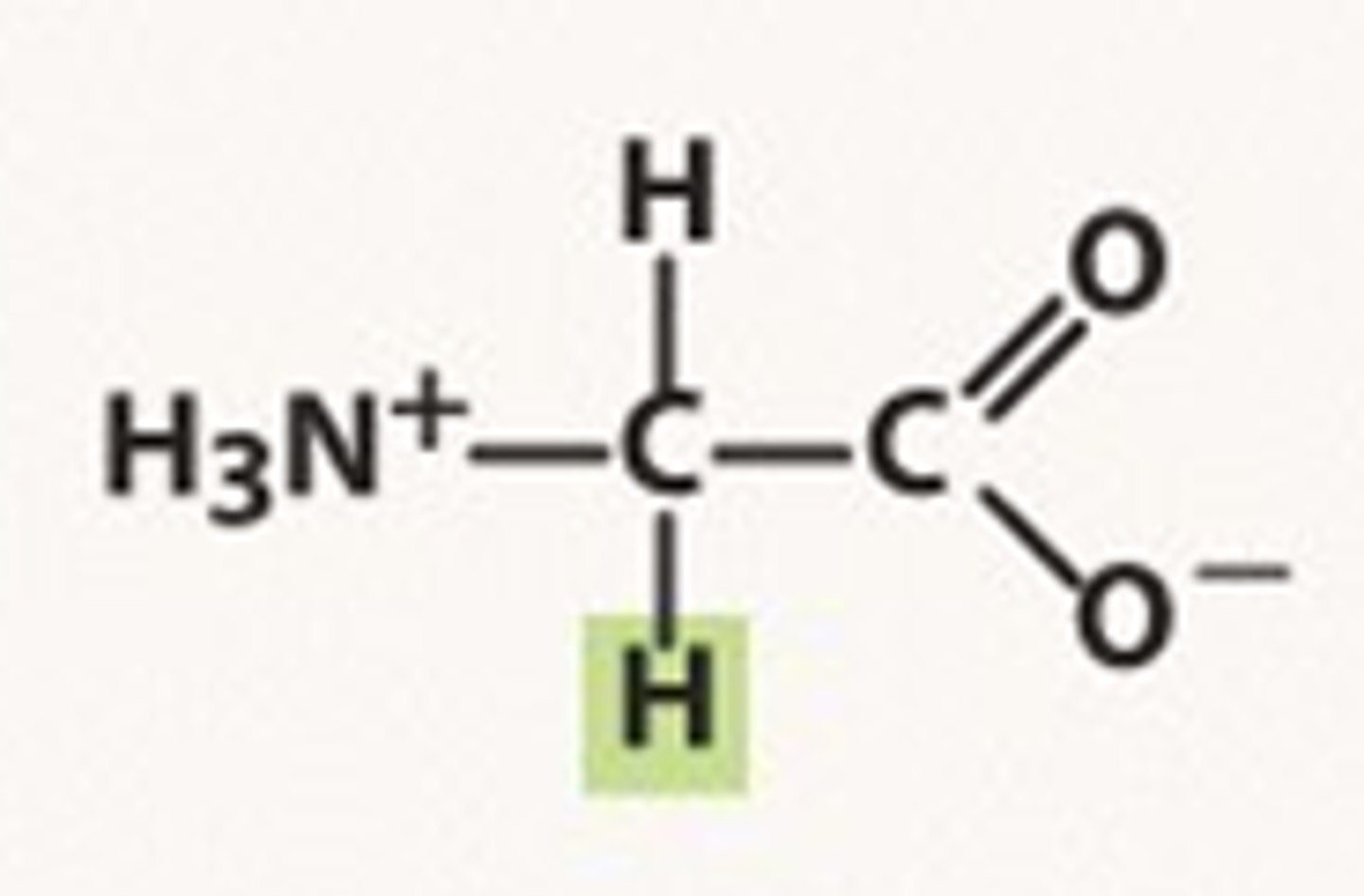
Isomer exception
glycine
ATP consists of
adenosine and a triphosphate tail of three phosphate groups
Adenosine =
adenine + ribose sugar
Where is C1 on the ATP molecule?
the first C is the closest one to the adenine group
4 types of macromolecules
carbohydrates - 4.3 cal/g
lipids - 9.3 cal/g
proteins -4.3 cal/g
nucleic acids
Phosphates in ATP are held together despite what forces?
electrostatic repulsion
Carbohydrates are..
the basis for synthesizing all other macromolecules. thus the first major macromolecule
Lipids are...
More efficient at storing energy
Made of HC chains which account for higher cal/g
Proteins are...
equally as efficient as carbs when storing energy
workhorses of the cell as they have multiple functions
Nucleic Acids are...
DNA, RNA -> storing info and gene expression
dehydration synthesis
A chemical reaction in which two molecules covalently bond to each other with the removal of a water molecule.
hydrolysis (hydrolytic cleavage)
Hydrolysis breaks a covalent bond by adding OH and H
When making a polysaccharide out of 20 molecules, how many water molecules do you lose?
19 water molecules are lost because n-1 (atoms - 1 = bonds)
Carbohydrates are made of which atoms?
C, O, H
Carbohydrates are usually polar or nonpolar?
polar, due to the presence of O
Monosaccharides
Simplest sugar: 3C
Most common:
5C: ribose, deoxyribose
6C: glucose
Carbohydrates usually have what structures?
Ring or linear
Threose Nucleic Acid
4C sugar thought to predate 5C sugars in DNA
Energy storage: starch vs glycogen
starch:
found in plants
glycogen:
main storage unit of CHO in animals. has significantly more branching than in starch
amylopectin vs amylose
both starches
amylopectin has more branching thus making it easier to break down than amylose
rice (amylose) is much harder to break down than potatoes (amylopectin)
Carbohydrates monomers:
Monosaccharide
The covalent bond that exists between carbohydrate monomers is
1-4 Glycosylic bond
(1st C of one glucose bonds with the 4th C of the other glucose)
Lipid monomers
triglycerides/triglycerols
Covalent bond between triglycerides (triglycerols)
Ester
Protein monomers
Amino acids
Covalent bond between amino acids
peptide bond
Nucleic acid monomers
nucleotides
Covalent bond between nucleotides
phosphodiester bond
Lipids are composed of which atoms?
H, C (hydrocarbon chains yo)
Lipids are typically polar or nonpolar?
Nonpolar due to HC chain. Insoluble in water
Saturated fat
SINGLE bonds
solid at room temp
unsaturated fats
more than 1 double bond present
liquid at room temp
High density lipoprotein (HDL)
blood fat that helps transport cholesterol out of the arteries, thereby protecting against heart disease
GOOD cholesterol
Low density lipoprotein (LDL)
blood fat that transports cholesterol to organs and tissues; excess amounts result in the accumulation of fatty deposits on artery walls
BAD cholesterol
Trans fats
has LDL
unsaturated fat
increasing heat turns cis-isomers into trans-isomers, becoming a more stable fat
cis isomer
an isomer of an alkene in which the hydrogen atoms in the double bond are on the same side
trans isomer
an isomer of an alkene in which similar groups in the double bond are on opposite sides
hydrogenation
adding H to cause double bond to become single bonds
omega 3 fatty acid
lowers BP, ensures good blood circulation, improves nerve impulses
the omega carbon is the the C FURTHEST from the functional group - ie the 3rd carbon
Phospholipid structure
Phosphate head
Glycerol connector
Two fatty acid tails
Amphipathic molecule
phosphate region - polar, hydrophilic
fatty acid chains - nonpolar
Phospholipids give the membrane...
semipermeability
because nonpolar lipid tails allow small hydrophobic molecules to pass thru
steroids
lipids characterized by a carbon skeleton consisting of four fused rings
Waxes
A type of lipid molecule consisting of one fatty acid linked to an alcohol; functions as a waterproof coating on many biological surfaces such as apples and other fruits.
terpenes
long-chain lipids that are components of many biologically important pigments
responsible for bright pink pigments in flamingoes
Examples of lipids
Steroids: 4C rings
Waxes: one long fatty acid chain
Fatty acid
A long carbon chain carboxylic acid. vary in length and in the number and location of double bonds; three fatty acids linked to a glycerol molecule form fat.
hydrolyzable lipids
waxes, triacylglycerols, phospholipids
nonhydrolyzable lipids
steroids, fat-soluble vitamins,
proteins consist of
C, H, N, O, and trace amounts of S
2 characteristics important to preserving life
the colligative properties of water
the existence of strong and short C=C bonds
2 definitions of proteins
workhorse of the cell (transport, hormones, enzymes)
relating genes as tools of gene expression
Amino acids are classified by their
functional group (R-group)
There are how many amino acids?
20
Protein polymer
polypeptides linked by peptide bond
Structure of amino acid without presence of H2O
central carbon, carboxyl group to the right (COOH), amine group to the left (NH2), hydrogen above and R group below
Structure of amino acid with the presence of H2O
central carbon, carboxyl group to the right (COO-), amine group to the left (NH3+), hydrogen above, R group below
Exception to isomerism
Glycine
has no D or L form because it has 2 hydrogens attached to the central C atom making it unable to form an enantiomer
The D form glucose
OH on right
L form glucose
OH on left
All protein translation begins with
methionine
(the amino acid that corresponds to the codon of AUG)
two types of amino acids
nonessential: made in the body (12 aa)
essential: consumed from external sources (8 aa)
peptide bonds are created by
dehydration synthesis/condensation reaction (water is lost to form a larger molecule)
Name of protein is related to...
the function of a protein
proteins must...to become active
fold and twist
where are the charges placed in a polypeptide chain?
the + charge on the left (amine group, NH3+)
the - charge on the right (carboxyl group, COO-)
myoglobin has what structure?
has 153 aa that form 1 polypeptide chain
primary, secondary, tertiary
hemoglobin has what structure?
has 2 alpha globins and 2 beta globin. have a total of 574 amino acids and 4 polypeptide chains
primary, secondary, tertiary, quaternary (bc 4 polypeptide chains)
primary structure
first level of protein structure
sequence of amino acids
never found in existence by itself but IS THE MOST IMPORTANT STRUCTURE
what is primary structure responsible for?
the sequence of amino acids determines structure and function of proteins thus making it the most important structure
sickle cell anemia
example of the importance of primary structure
mutation in the primary structure of beta globin (change of one nucleotide from GLU to VAL) affects hemoglobin. Ultimately resulting in sickle rather than spherical shape of red blood cells
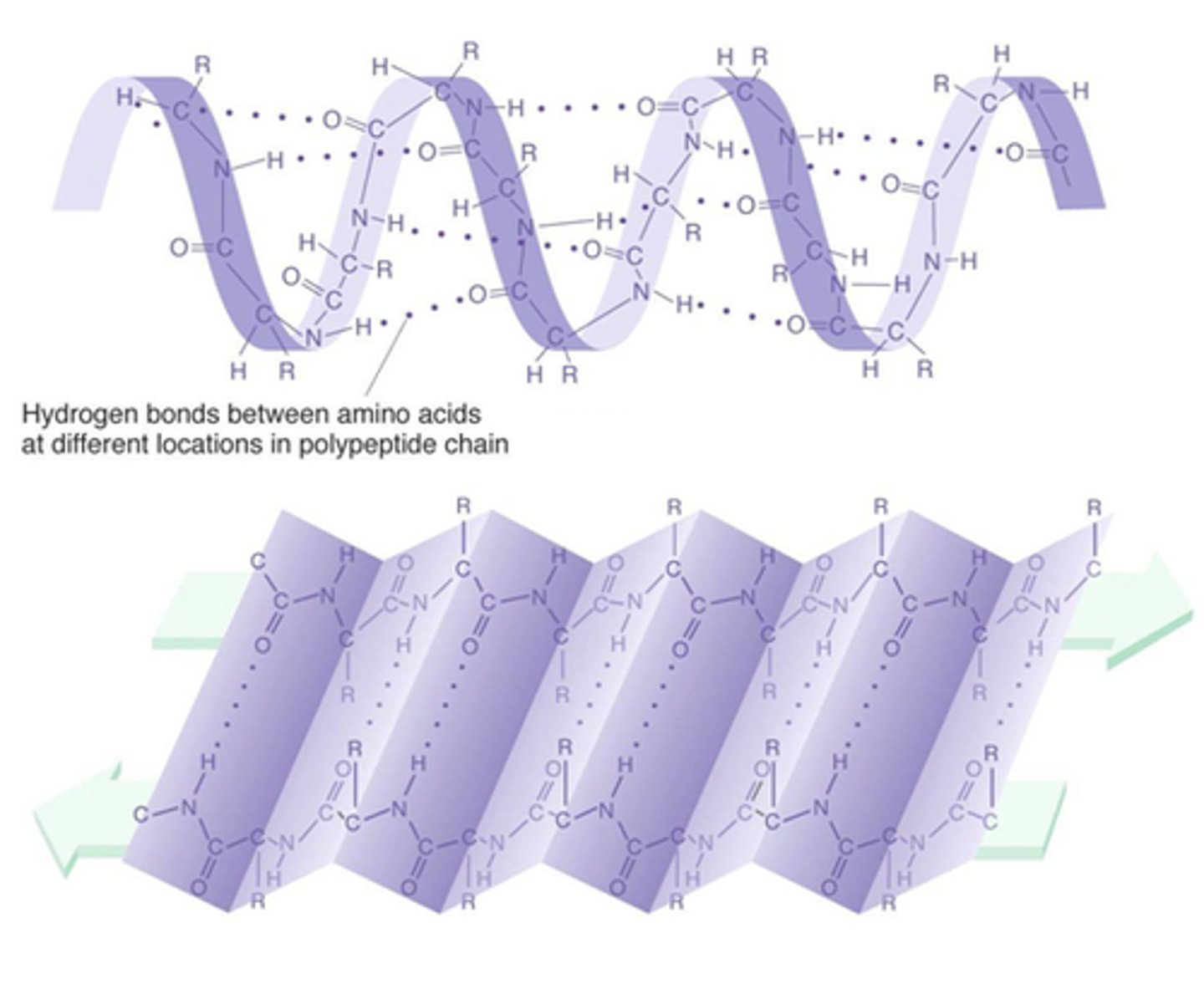
Secondary Structure
held together by only H-bonds (intermolecular H-bonds between distant amine/carboxyl groups pushes amino acids outwards, forming alpha helical structure)
Motif
part of secondary structure
shape that isn't helical or pleated sheet
specific shape that is important to function
ie. enzyme active site specificity
The strength of a protein is determined by which structure?
secondary structure
components of secondary structure
alpha helices, beta pleated sheets, and motifs (super secondary structures)
tertiary structure
The third level of protein structure; the overall, three-dimensional shape of a polypeptide due to interactions of the R groups of the amino acids making up the chain.
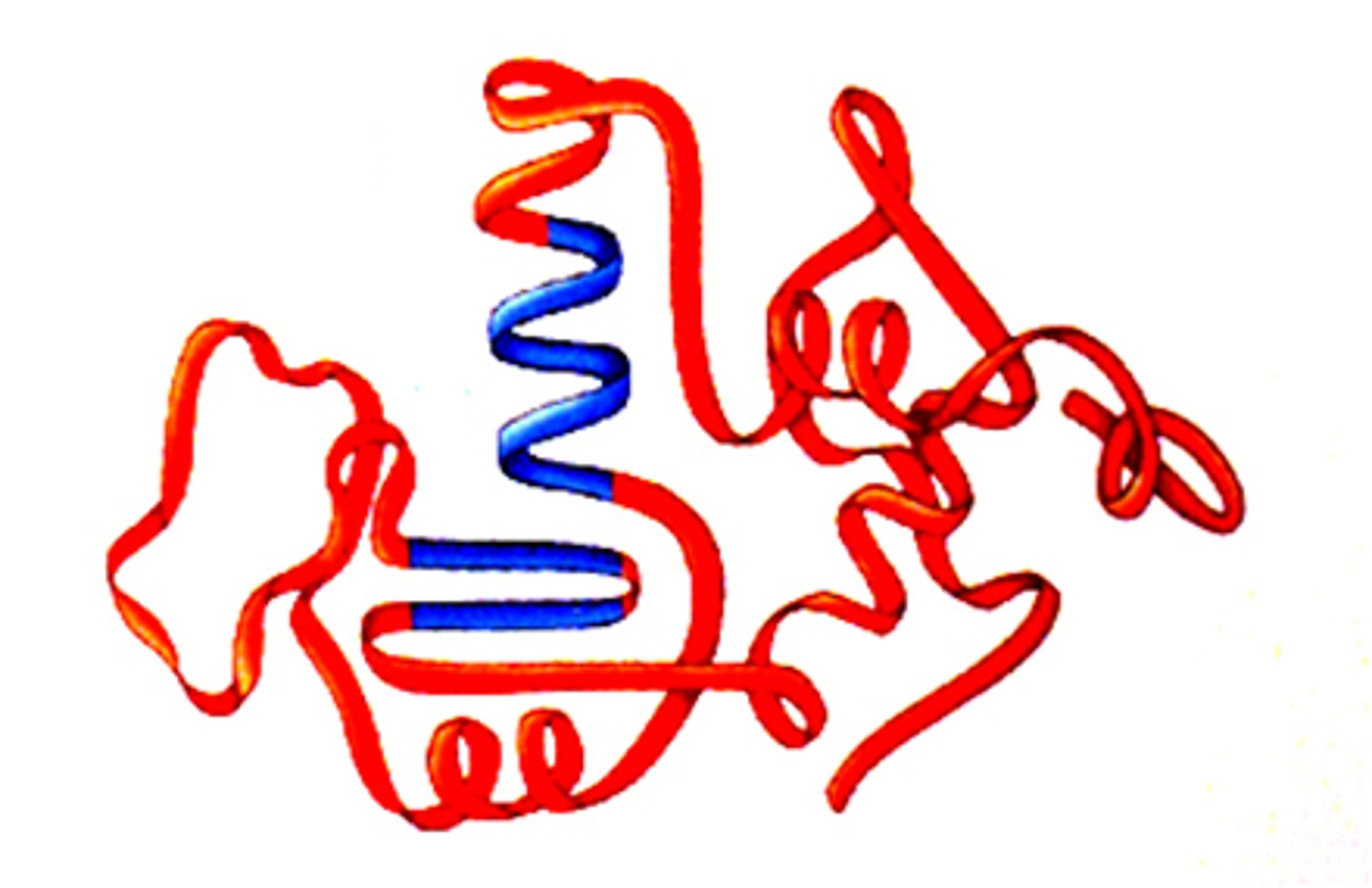
components of tertiary structure
H-bonds and ionic polar bonds
hydrophobic exclusion
van der waals forces
disulfide bridges
hydrophobic exclusion
repulsion when interacting with water - nonpolar
disulfide bridges
the ONLY covalent bonds that extend from amino acids that contain sulfur
stabilize tertiary structure and quaternary structure
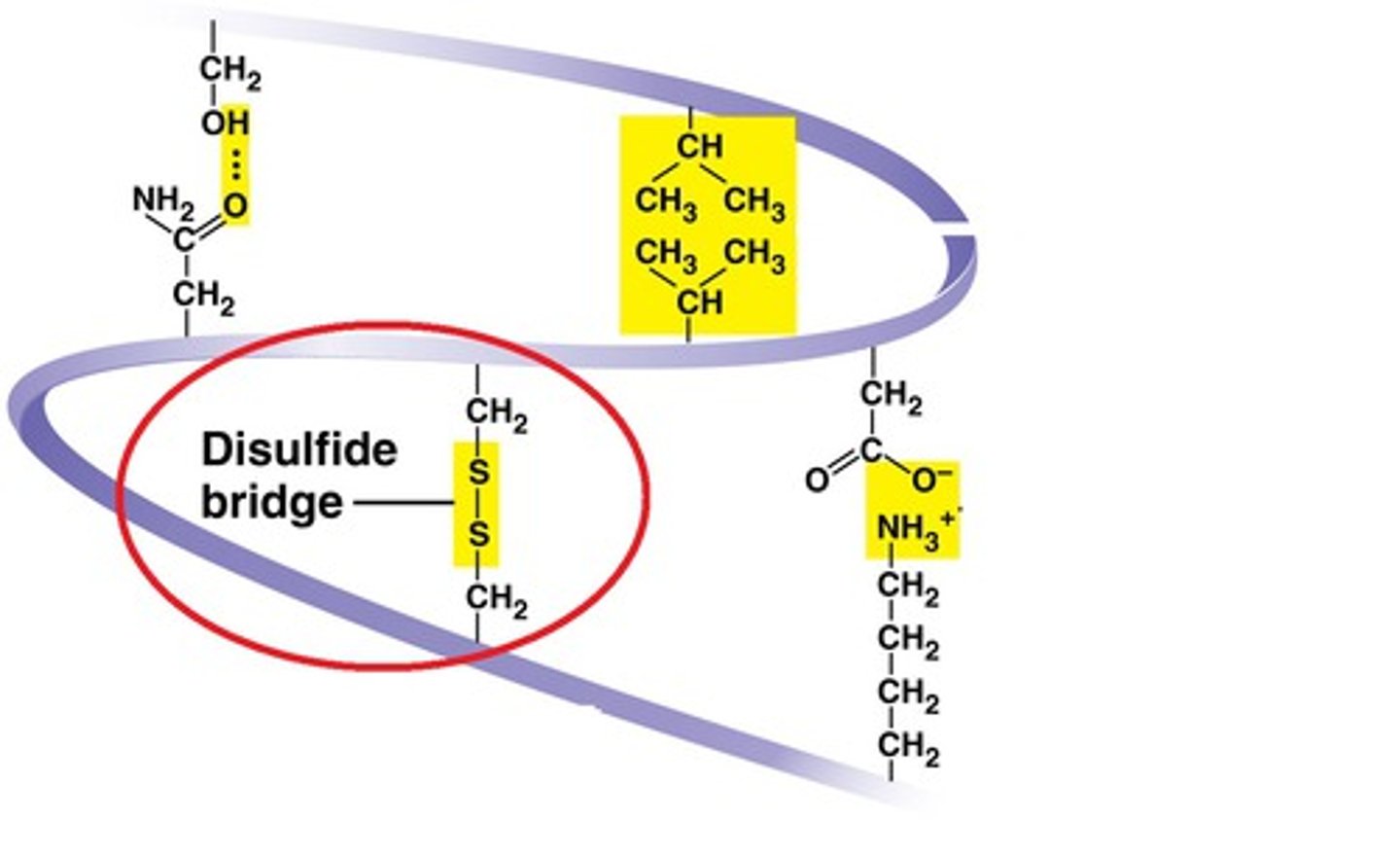
Do all polypeptides exhibit tertiary structure?
Yes
Do all proteins exhibit tertiary structure?
No. Some proteins do not have tertiary structure (disordered proteins)
A protein or polypeptide has 3D structure, what does this imply?
the protein or polypeptide has tertiary structure
quaternary structure
The fourth level of protein structure; the shape resulting from the association of two or more polypeptide subunits.
tertiary polypeptide is not yet functional, what must it do to become a functional protein?
combine with another polypeptide(s), attain quarternary structure to become functional
Domains of proteins
quarternary proteins contain several domains
domains contain their own motifs
each domain is involved in distinct biological function. proteins that share domain share same function
Active sites
shape and chemical composition only allows binding of certain molecules
this shape and composition comes from amino acids
While proteins may have different functions and shapes, what is one thing they have in common?
Domains/motifs. Proteins MUST bind to DNA as as genetic tool. To bind to DNA, they all req the same active site/domain/motif
Denaturation
protein unfolding, destruction of structure
any protein can undergo denaturation, regardless of structure (both myoglobin and hemoglobin can denature)
results in metabolic disease
protein dissociation
only affects proteins with multiple polypeptides/quaternary structure
(myoglobin cannot undergo protein dissociation but hemoglobin can)
Is it more energy efficient to have forces of attraction or covalent bonds in between proteins?
Forces of attraction exist between proteins (enzyme-substrate rxns)
no disulfide bridges (covalent bond) during protein-protein interactions (disulfide bridges req more permanent situations and are more difficult to break)
Is it more energy efficient to have forces of attraction or covalent bonds in between polypeptides?
Covalent peptide bonds exist in between polypeptides, thus are more energy efficient
nucleic acids contain which elements?
C, N, O, P, H