CH 2 Polyatomics, Acids, Common Names, Alcohols
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Hydroxide
OH-
Hydronium
H3O+
Ammonium
NH4+
Nitrate
NO3-
Nitrite
NO2-
Perchlorate
ClO4-
Chlorate
ClO3-
Chlorite
ClO2-
Hypochlorite
ClO-
Carbonate
CO32-
Bicarbonate
HCO3-
Cyanide
CN-
Sulfate
SO42-
Sulfite
SO32-
Phosphate
PO43-
Hydrogen Phosphate
HPO42-
Dihydrogen Phosphate
H2PO4-
Phosphite
PO33-
Acetate
CH3COO-
Permanganate
MnO4-
Water
H2O
Ammonia
NH3
Hydrogen Sulfate
H2S
Methane
CH4
Ethane
CH3CH3
Propane
CH3CH2CH3
Hydrofluoric acid
HF
Hydrochloric acid
HCl
Hydrobromic acid
HBr
Hydroiodic acid
HI
Chloric acid
HClO3
Perchloric acid
HClO4
Nitric Acid
HNO3
Sulfuric Acid
H2SO4
Aceteic acid
CH3COOH
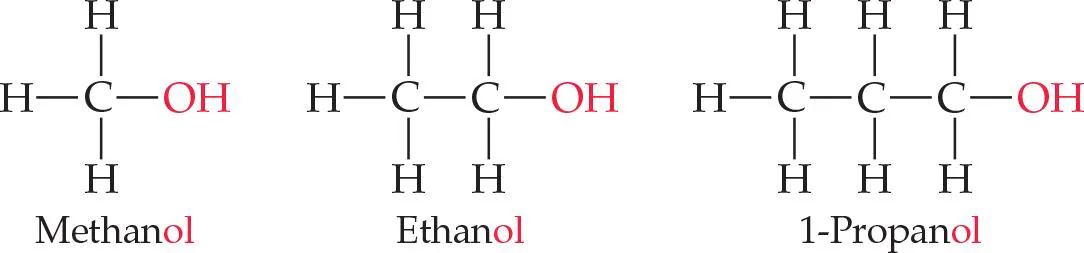
How do alcohol functional groups work for:
Methanol
Ethanol
1-Propane
2-Propane
When adding an -OH functional group to these molecules, simply add “ol” in the back of it.” the OH functional group replaces one H.
Methanol
Ethanol
Depending on the location of the functional group being on a specific carbon atom, it has a #- in front of it.
1-Propanol
connected to either the 1st or 3rd carbon atom
there is NO 3-Propanol because if you just turn the molecule around it is the same as 1-Propanol
2-Propanol
connected to the 2nd (middle) carbon atom