National 5 Chemistry - Chemical Analysis + Techniques
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Draw the apparatus that would allow a soluble gas to be removed from a mixture of gases.
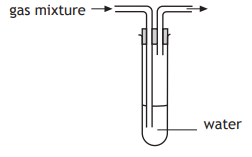

What is this?
A conical flask

What is this?
A beaker

What is this?
A measuring cylinder

What is this?
A test tube

What is this?
A funnel

What is this?
A dropper

What is this?
A pipette
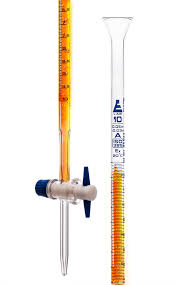
What is this?
A burette
Explain the process of collecting a gas produced in a reaction by using a gas syringe.
As the reaction takes place, the gas is collected directly in the syringe; the syringe plunger moves outwards as gas is produced
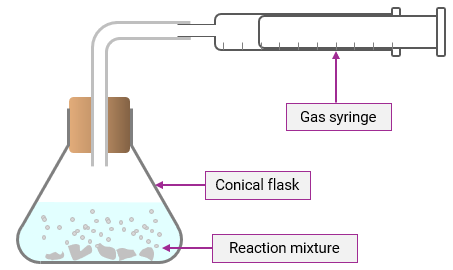
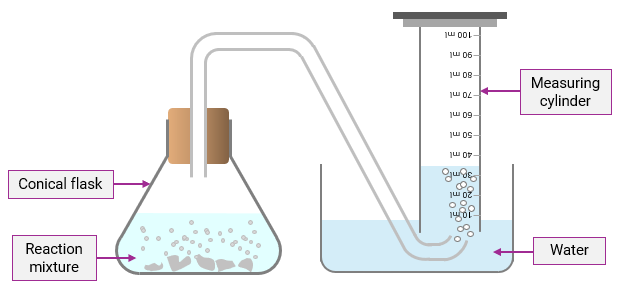
Explain the process of collecting a gas produced in a reaction by using a measuring cylinder.
The gas produces bubbles and displaces water; the gas must be insoluble in water for this to work effectively
State the term used to describe a substance that neutralises an acid.
A base
Name the compound that is produced in all neutralisation reactions.
Water
How do you know when an acid has been completely neutralised?
You know an acid is neutralized when the solution reaches a pH of 7 (neutral), indicated by a pH meter or a specific color change with an indicator - or if it has been reacted with a metal carbonate, when there is no more fizzing/bubbles
What is the test for oxygen?
It relights a glowing splint
What is the test for hydrogen?
It burns with a squeaky pop
What is the test for carbon dioxide?
It turns limewater cloudy
How can metals be identified based on reactivity?
React the given metals with water or acid, observe how vigorously they react, and arrange them into a reactivity series
How can metals be identified based on flame colours?
Use tongs to hold a piece of the metal above a Bunsen flame and observe what colour of flame is produced; then use the data booklet to identify the metal
What is a standard solution?
A solution of accurately known concentration