SN2
1/113
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
114 Terms
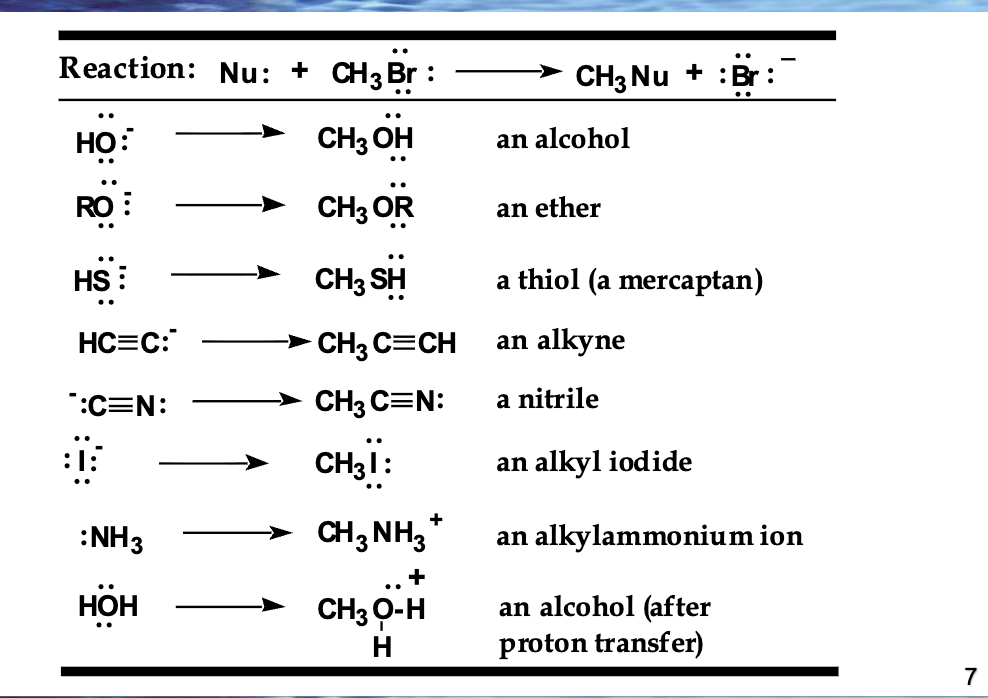
If a halogen replaces a hydrogen, you get a …. (A)
haloalkane also known as alkyl halides
what are the steps to naming a haloalkane?
Find the parent alkane (the one closest to the halogen)
Number the chain so the halogen gets the lowest number
Add the halogen prefix + number before the alkane name
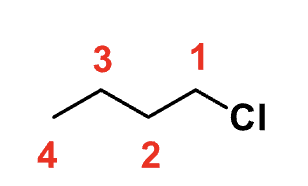
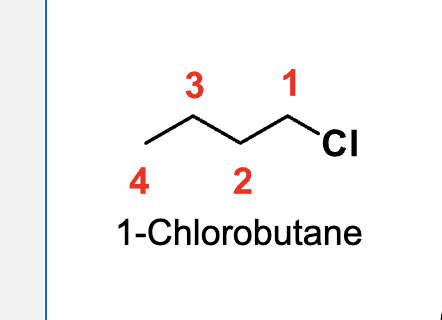
?
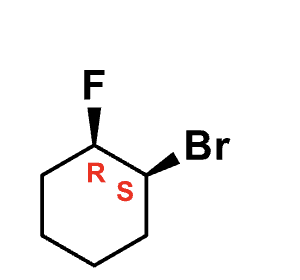
name this
1S,2R)-1-Bromo-2-
fluorocyclohexane
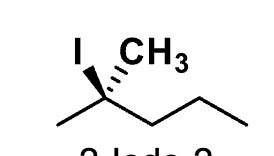
name this
2-Iodo-2-
methylpentane
What is a C–X bond?
Back: A bond between carbon and a halogen (F, Cl, Br, or I).
Front: Why is the C–X bond polarized?
Back: Because halogens are more electronegative and pull electrons toward themselves.
Front: In a C–X bond, which atom is δ⁺ and which is δ⁻?
Back: Carbon is δ⁺, halogen is δ⁻.
Front: Which way does the dipole arrow point in a C–X bond?
Back: Toward the halogen.
Front: In alkyl halides, where do nucleophiles attack?
Back: The carbon attached to the halogen.
Front: What does R–X represent?
Back: An alkyl halide (a carbon chain bonded to a halogen).
Front: How does C–X bond length change from F → I?
Back: It increases (gets longer).
Front: How does C–X bond strength change from F → I?
Back: It decreases (gets weaker).
Front: How do boiling points change from R–F to R–I?
Back: They increase.
Front: What causes boiling points of R–X to increase down the group?
Back: Increased size and polarizability → stronger London dispersion forces.
Front: What is a nucleophile?
Back: A molecule or ion that donates a pair of electrons (Lewis base).
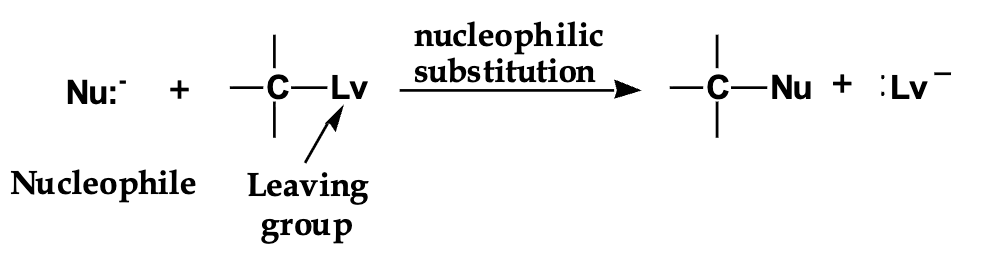
what is this? and explain it?
Back: A reaction where a nucleophile replaces a leaving group on a carbon.
Front: Why do nucleophiles react?
Back: They have extra electrons and want to donate them.
Front: Where does nucleophilic substitution happen?
Back: At an sp³ (tetrahedral) carbon.
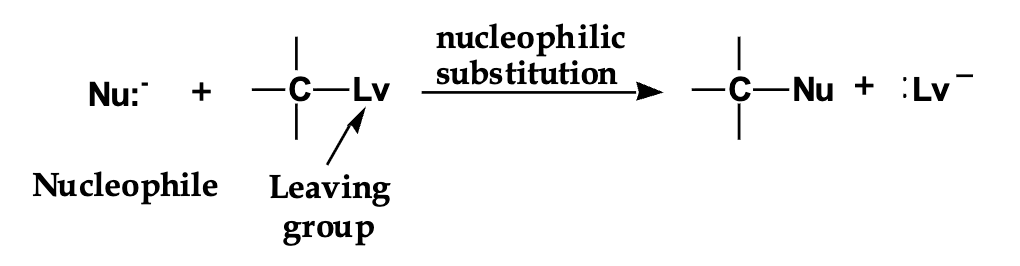
draw the arrows
Front: Where does the first curved arrow start and end?
-Back: Starts at the lone pair on Nu: and points to the carbon.
Front: Where is the leaving group before the reaction?
-Back: Directly bonded to the carbon (written as C–Lv).
Front: Where does the second curved arrow start and end?
-Back: Starts at the C–Lv bond and points to Lv.
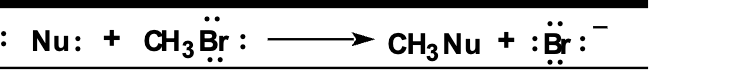
draw the arrows (DONT ERASE)
Front: Where does the FIRST curved arrow start and end?
Back: Starts at the lone pair on Nu: and points to the carbon of CH₃Br.
Front: Where does the SECOND curved arrow start and end?
Back: Starts at the C–Br bond and points to Br.
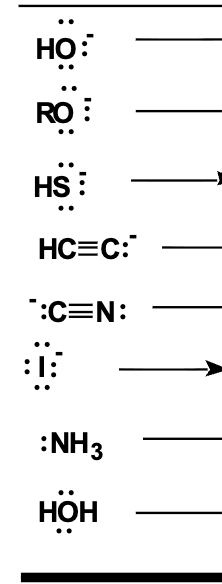
use this to draw all the finish all the reactions
(look at the question before)
Front: In SN2, when does bond formation occur relative to bond breaking?
Back: At the same time (simultaneously).
Front: What does the “2” in SN2 stand for?
Back: Bimolecular (two species involved in the rate-determining step).
Front: Which two species are involved in SN2?
Back: The nucleophile and the substrate (R–X).
Front: Does SN2 have an intermediate?
No
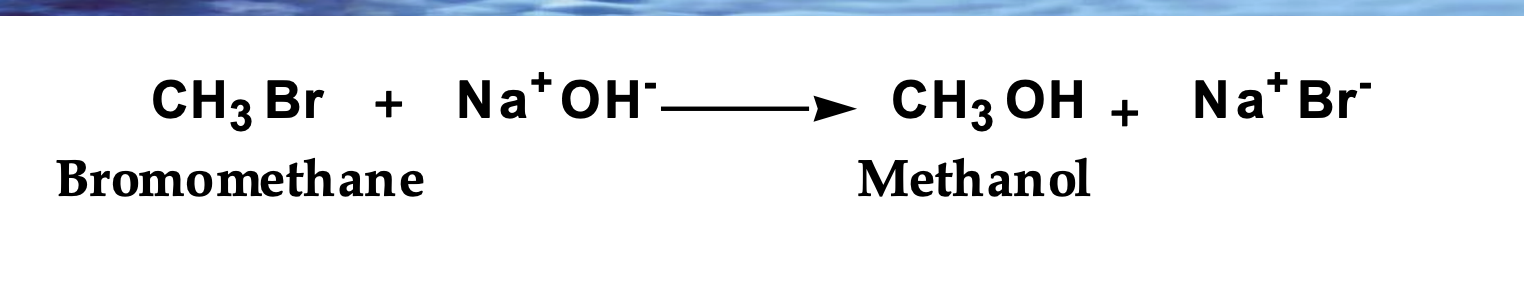
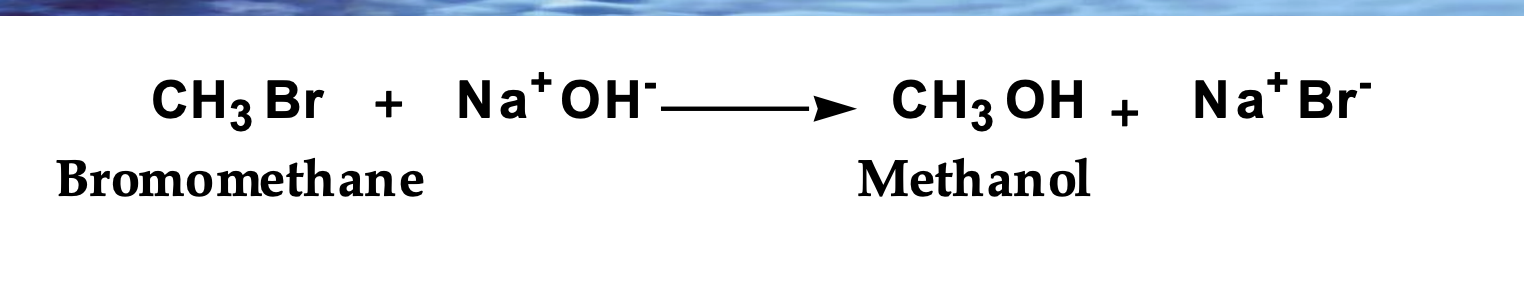
Show the mechanism
Rate = k[CH₃Br][OH⁻]
Na⁺ is not in the rate law → spectator
![<ul><li><p>Rate = k[CH₃Br][OH⁻]</p></li><li><p>Na⁺ is <strong>not</strong> in the rate law → <strong>spectator</strong></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ab9977c3-885a-4c7f-94a2-9d3dadb0dbd1.png)
draw the reaction coordinate diagram for this reaction
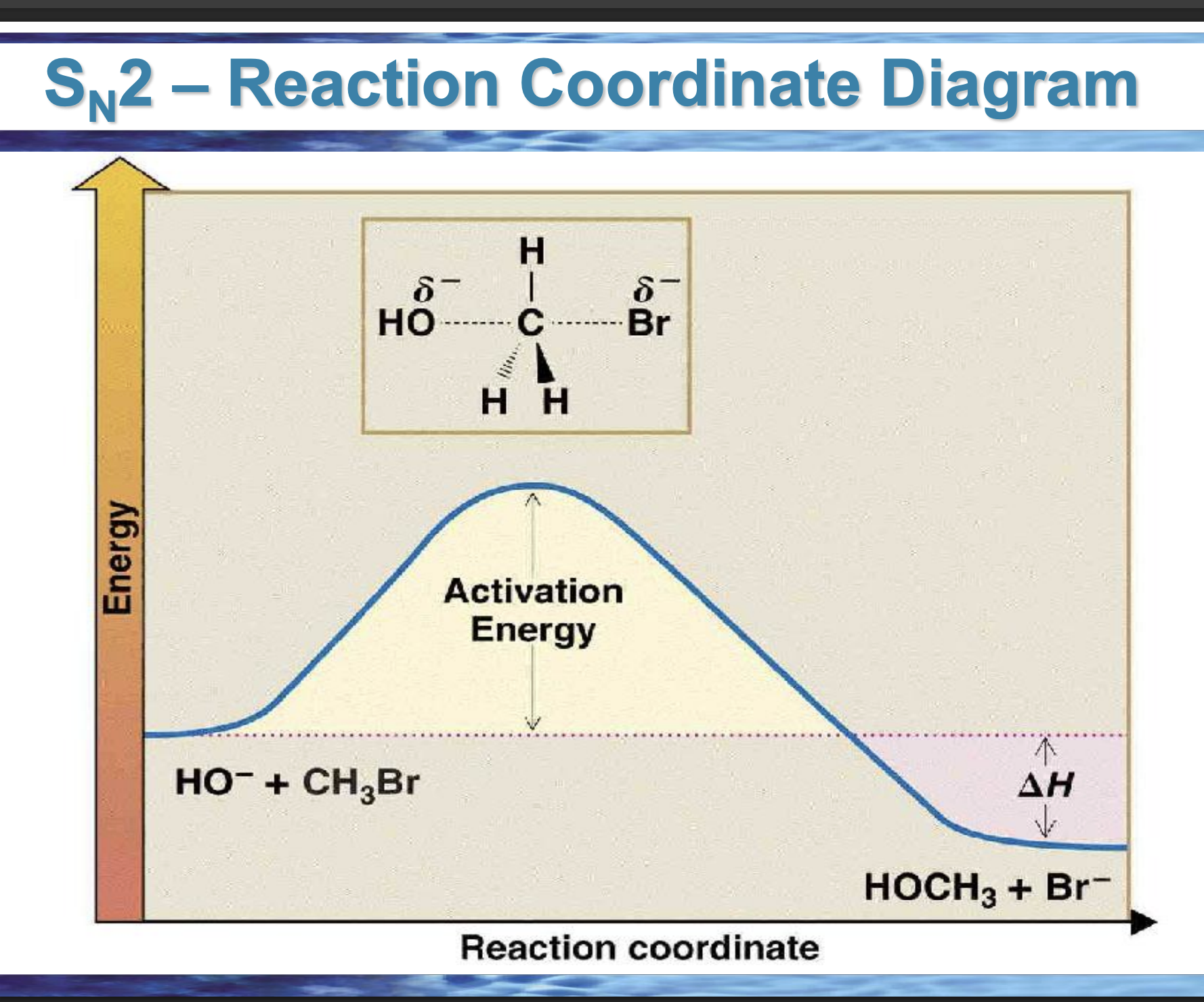
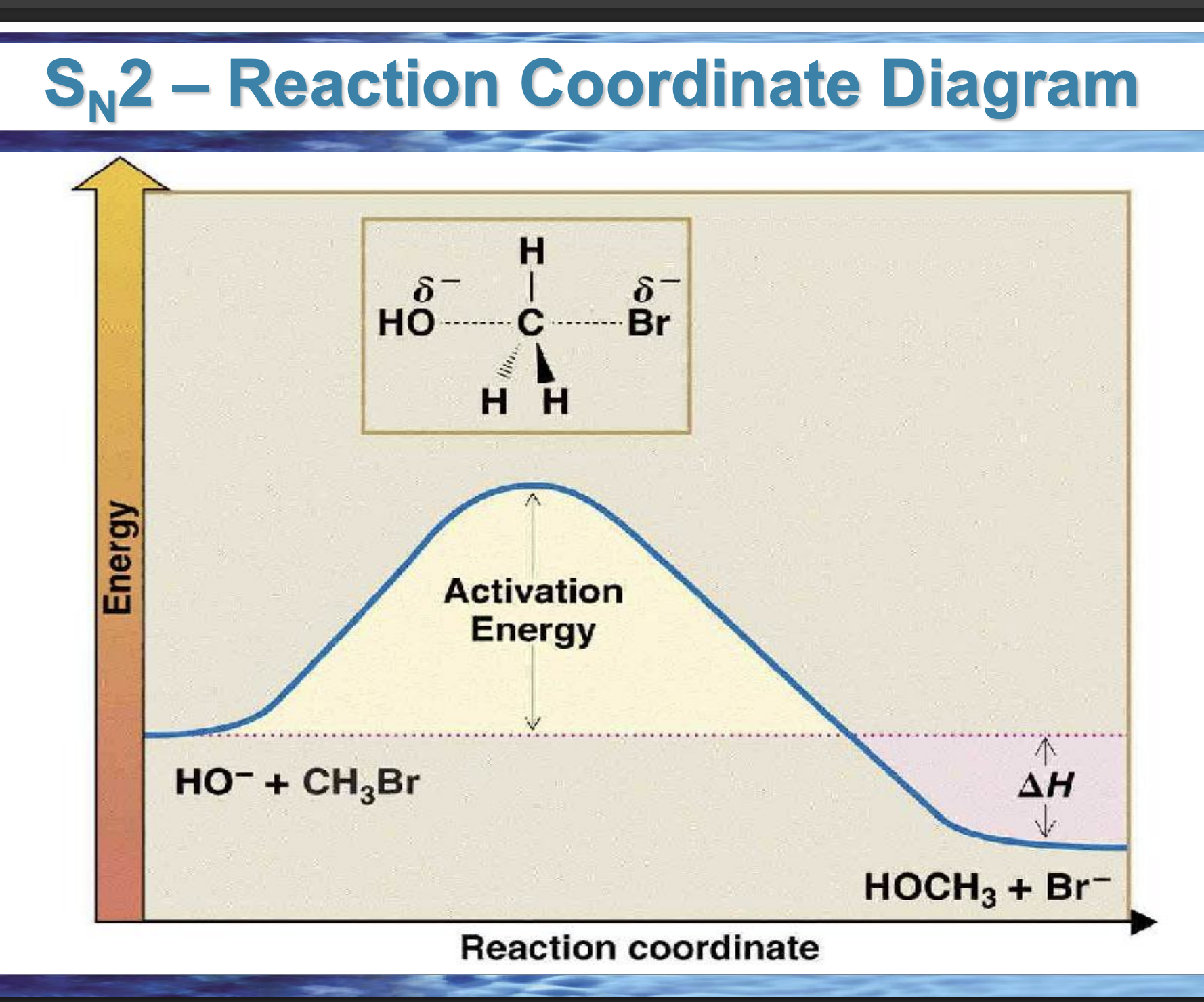
Front: What does the top of the hill represent?
Back: The transition state.
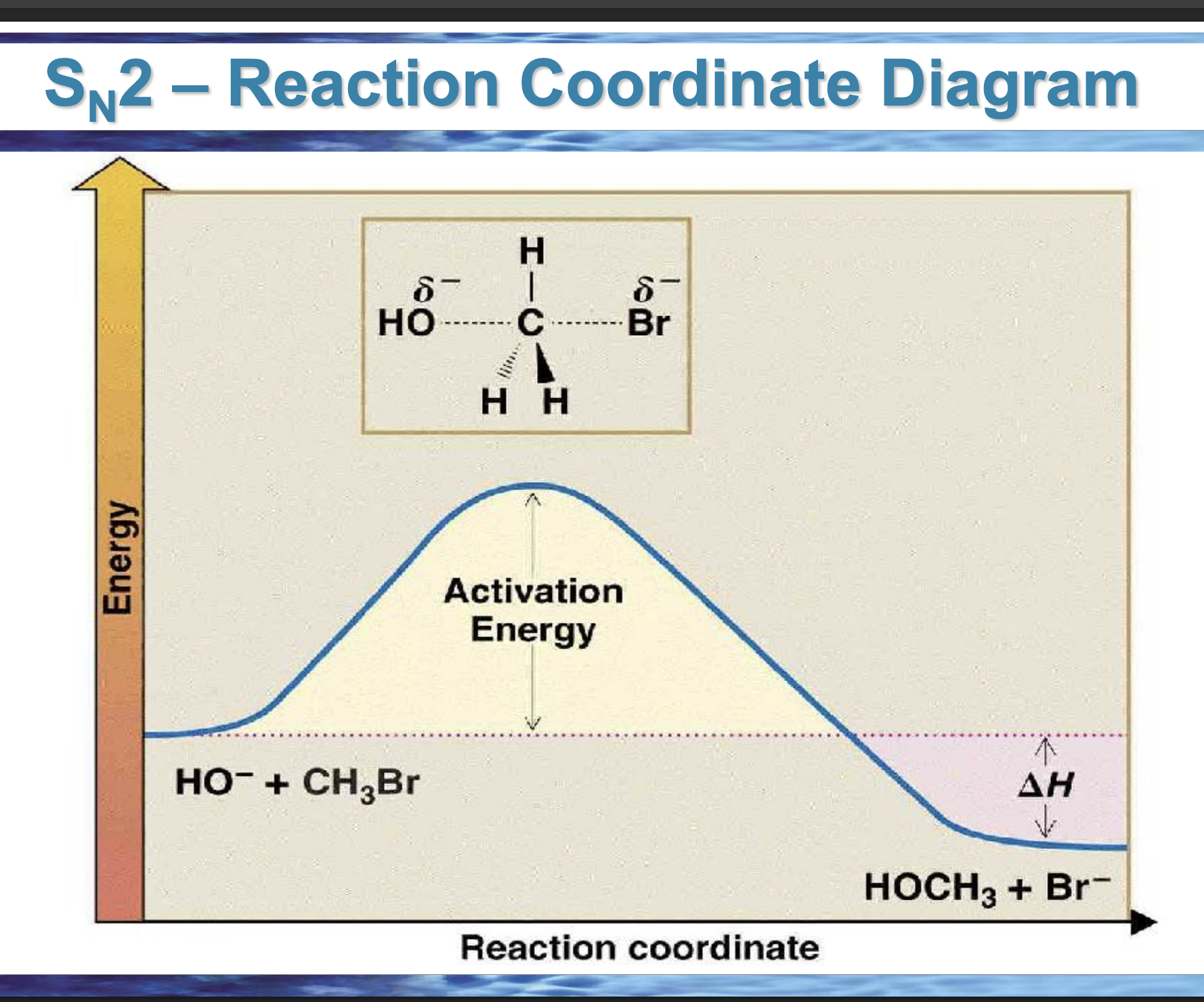
Front: What bonds are changing at the SN2 transition state?
Back: C–Nu forming and C–Lv breaking at the same time.
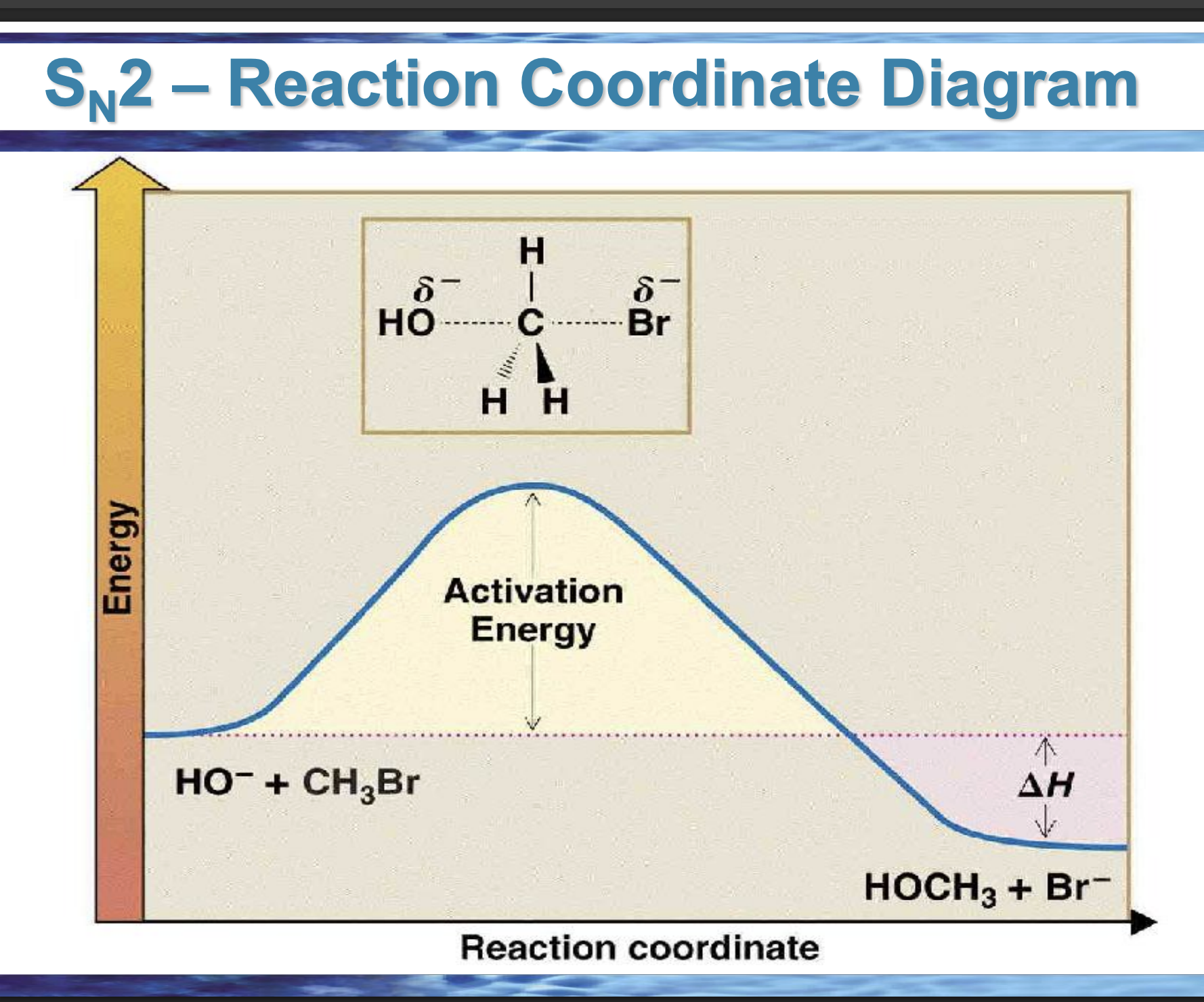
Front: What does ΔH measure?
Back: Energy difference between products and reactants.
Front: If products are lower in energy than reactants, what is ΔH?
Back: Negative (exothermic).
Front: In an endergonic reaction, are products higher or lower in energy?
Higher
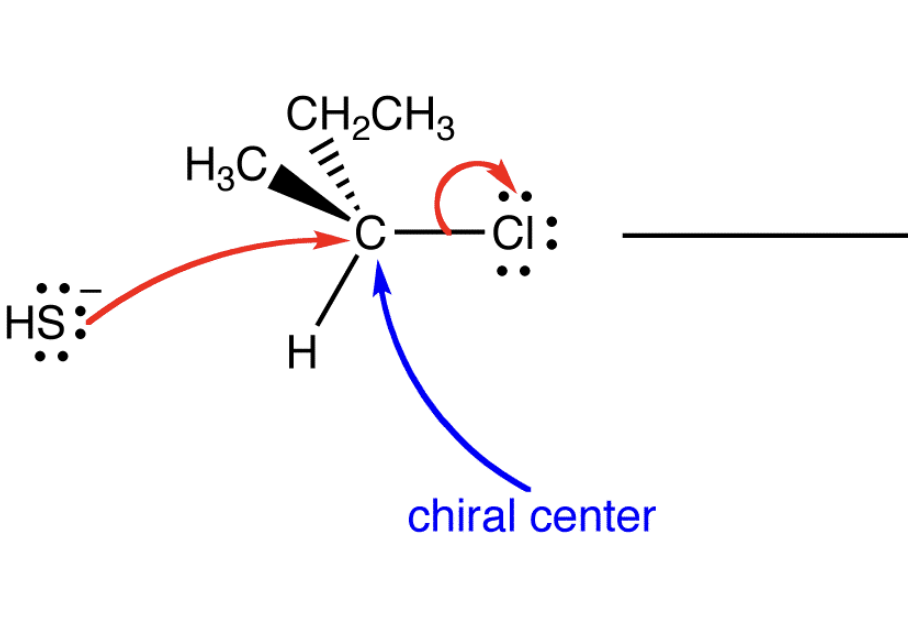
draw the inversion of the chiral center
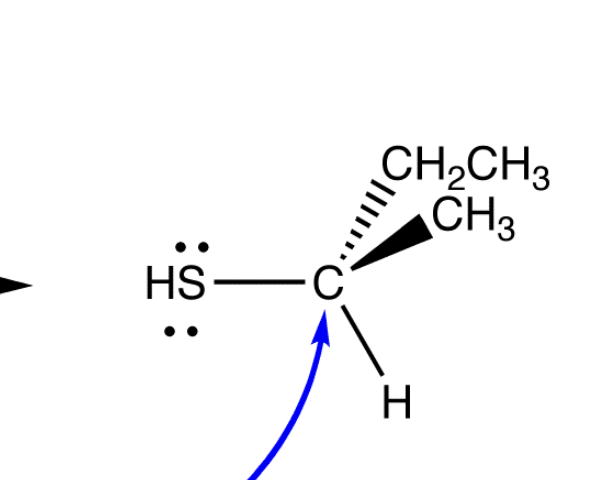
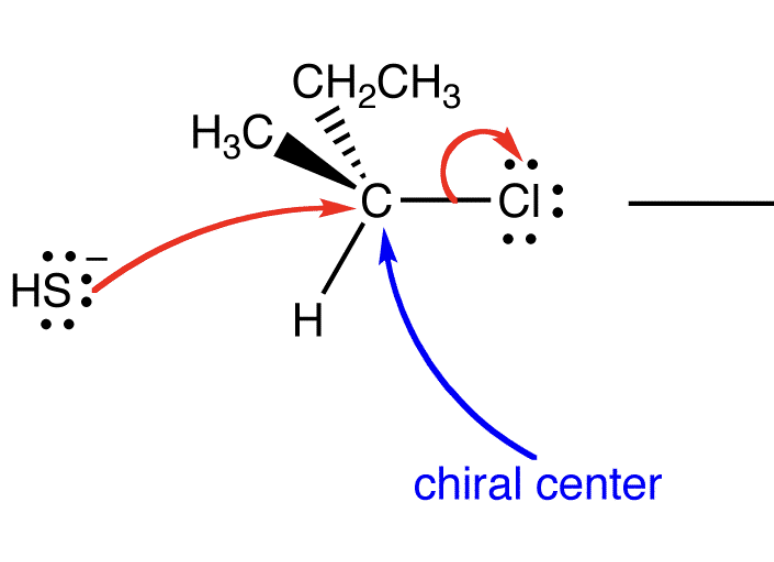
draw the sn2 reaction mechanism
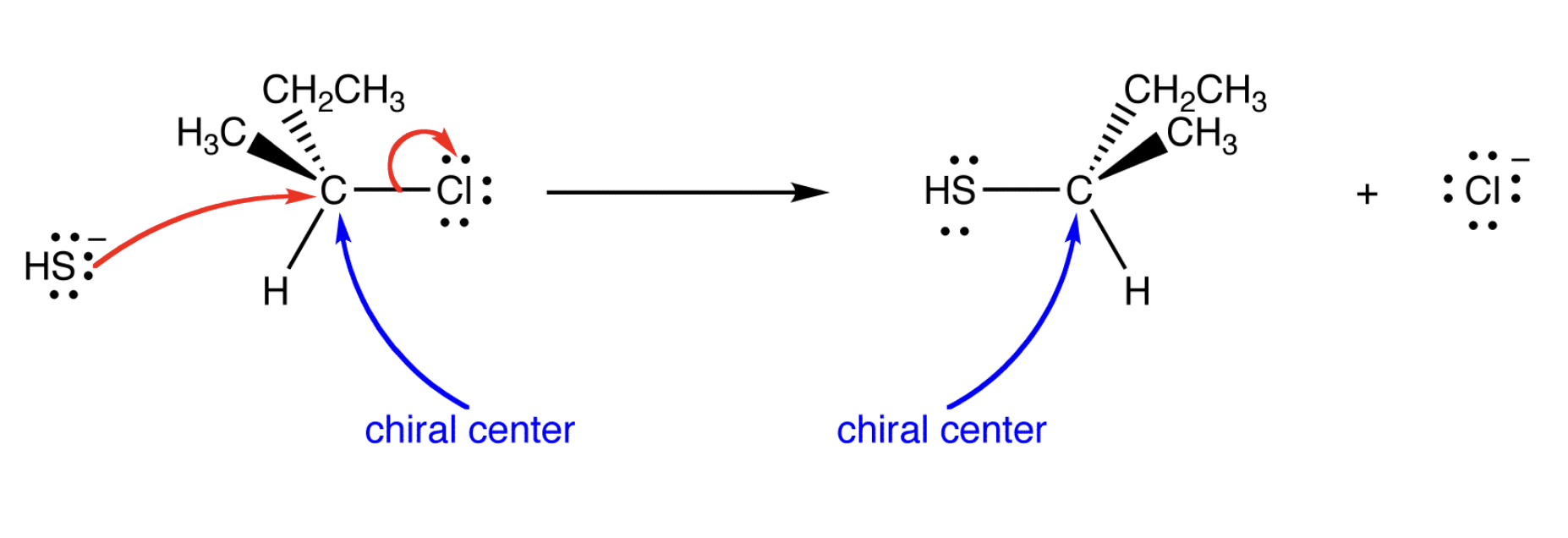
Front: What causes inversion in SN2?
Back: Backside attack of the nucleophile.
Front: What happens to the 3D arrangement at the carbon after SN2?
it flips/inverts
Front: What ALWAYS inverts in SN2?
Back: The relative configuration at the carbon.
Front: Does SN1 also give inversion?
Back: No (SN1 gives racemization).
Front: Why is inversion unavoidable in SN2?
Back: The nucleophile must attack from the backside as the leaving group leaves.
Front: What makes a good leaving group?
Back: A stable anion.
Front: Good leaving groups are conjugate bases of what?
Strong acids
Front: Rank halides by leaving group ability.
Back: I⁻ > Br⁻ > Cl⁻ > F⁻.
Front: Why is F⁻ a poor leaving group?
Back: It is small and unstable as an anion.
Front: Are strong bases good leaving groups?
NO
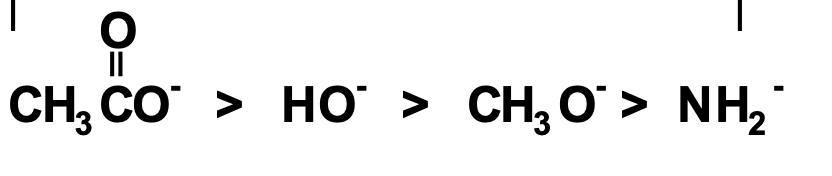
are these good leaving groups?
no they rarely function
They are ordered from best leaving group → worst leaving group.
Front: Why is TsCl used on alcohols?
Back: To convert OH into a good leaving group (OTs).
Front: Is OTs⁻ a strong or weak base?
Back: Very weak base.
Front: What does “steric hindrance” mean?
Back: Physical crowding that blocks nucleophile approach.
Front: Rank substrates by SN2 reactivity.
Back: Methyl > Primary > Secondary >> Tertiary.
Front: Which substrate does NOT undergo SN2?
Back: Tertiary.
Front: Why is SN2 negligible for tertiary substrates?
Back: Too much steric hindrance blocks backside attack.
Front: What is β-branching?
Back: Alkyl substitution on the carbon adjacent to the reacting carbon.
Front: Which carbon is the β-carbon?
Back: The carbon next to the one bonded to the leaving group.
Front: How does β-branching affect SN2?
Back: It slows SN2 by blocking backside attack.
Front: What happens to SN2 rate as β-branching increases?
Back: It decreases dramatically.
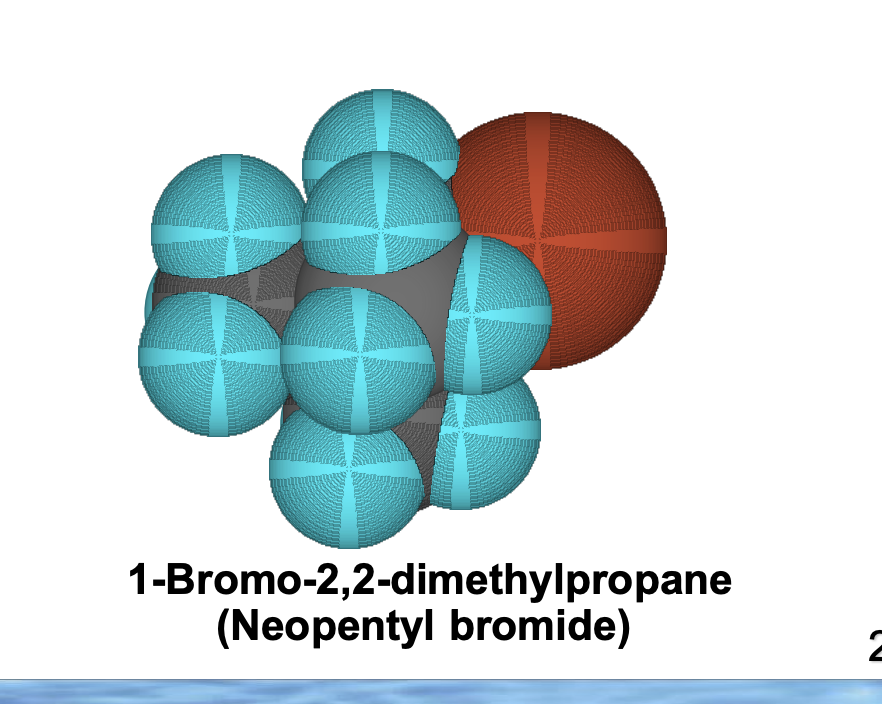
Front: Why is neopentyl bromide bad for SN2?
Back: Three β-branches cause extreme steric hindrance.
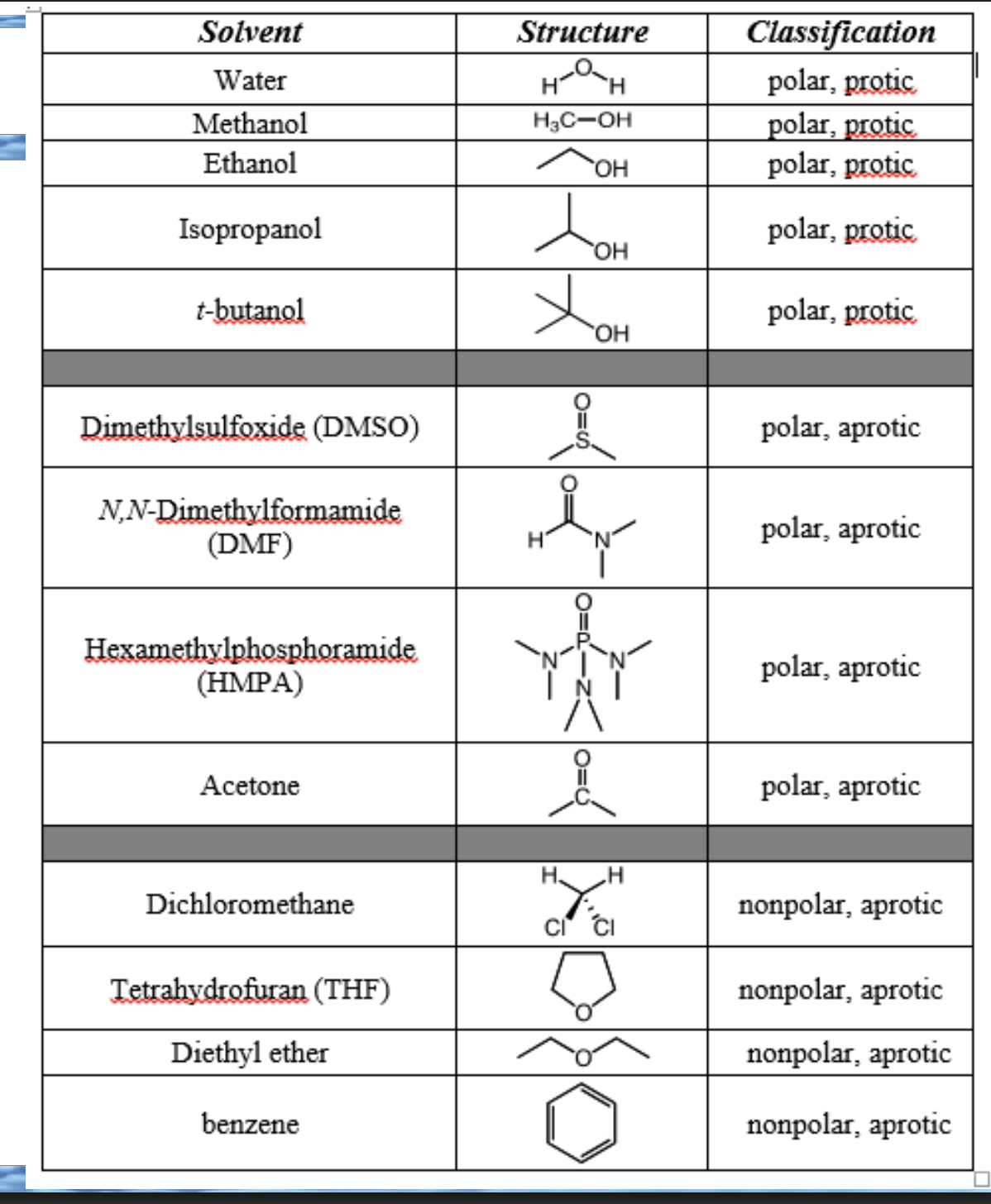
Front: What is a protic solvent?
Back: A solvent that can donate a hydrogen bond.
Front: What structural feature makes a solvent protic?
Back: An H bonded to O or N.
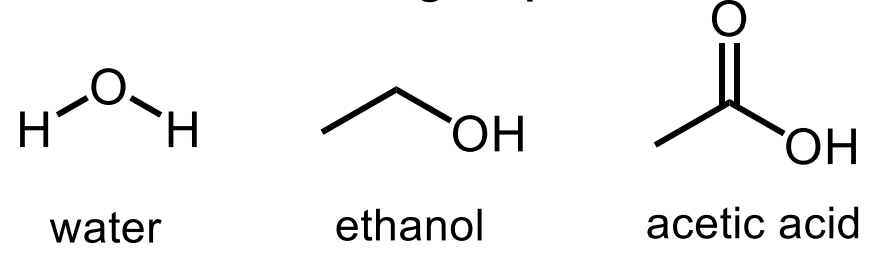
what are these?
protic solvents
Front: Why are aprotic solvents not hydrogen-bond donors?
Back: They have no H bonded to O or N.
Front: Can a solvent be polar and aprotic?
Yes
The molecule contains electronegative atoms (like O, N, S) → polarity
All hydrogens are bonded to carbon (or no hydrogens at all) → aprotic
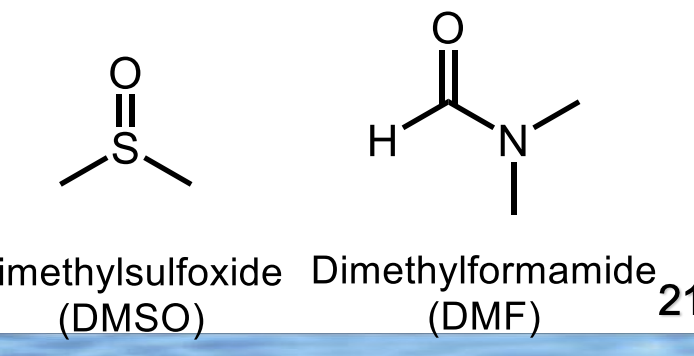
what are these?
aprotic solvents
what does solvation mean?
Solute = the thing you dissolve
Solvent = the liquid doing the dissolving
Solvation is when the solvent wraps around the solute and stabilizes it.
positive ions → solvent points its positive or negative end at them
negative ions → solvent points its positive or negative end at them
Positive ions → solvent points its negative end at them
Negative ions → solvent points its positive end at them
Solvation can slow down or weaken nucleophiles, depending on the solvent. how?
Polar protic solvents strongly solvate (hug) nucleophiles → nucleophile weaker
Polar aprotic solvents weakly solvate → nucleophile stronger
Front: What must a nucleophile do before attacking in SN2?
Back: Escape its solvation shell.
Front: What does strong solvation do to SN2 rate?
Back: Slows it down.
Front: Why does weak solvation speed up SN2?
Back: Less energy is needed to free the nucleophile.
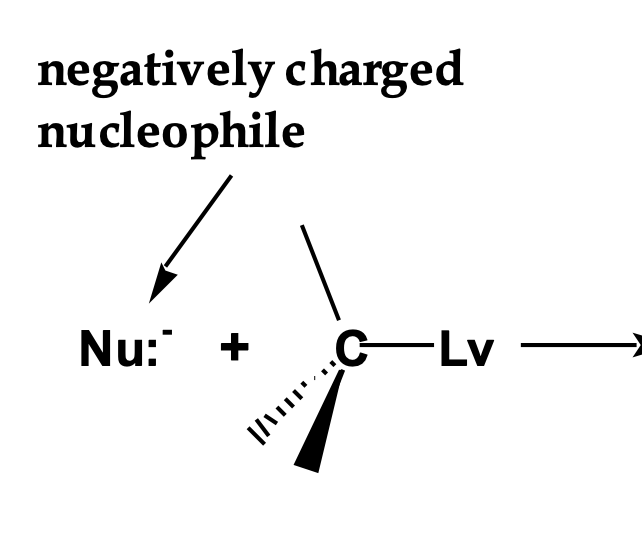
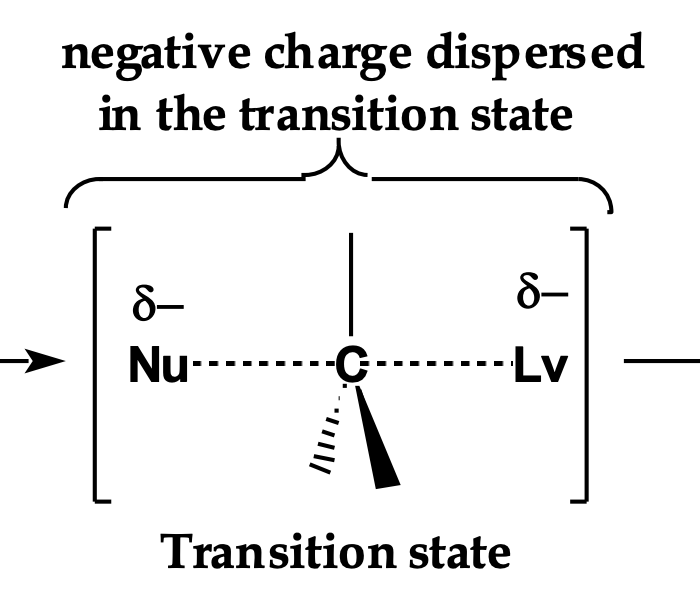
Front: How is negative charge distributed in the SN2 transition state? draw it!
Back: Spread between the nucleophile and leaving group.
Front: Do protic solvents solvate nucleophiles strongly or weakly?
Back: Strongly — protic solvents tightly surround nucleophiles with hydrogen bonding.
Front: Do polar aprotic solvents strongly or weakly solvate nucleophiles?
Back: Weakly — they do not hydrogen-bond to nucleophiles.
Front: Which solvent type gives faster SN2: protic or polar aprotic?
Back: Polar aprotic.
draw it
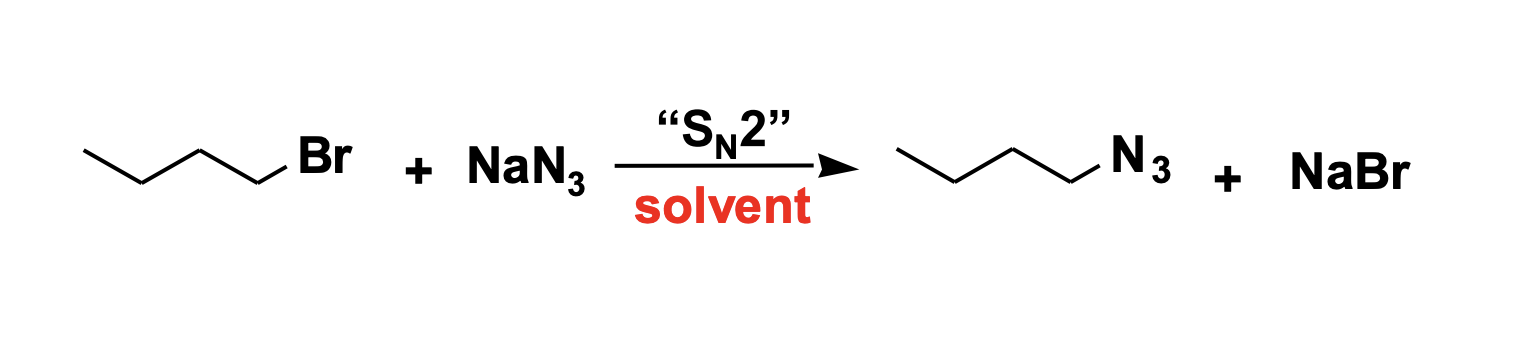
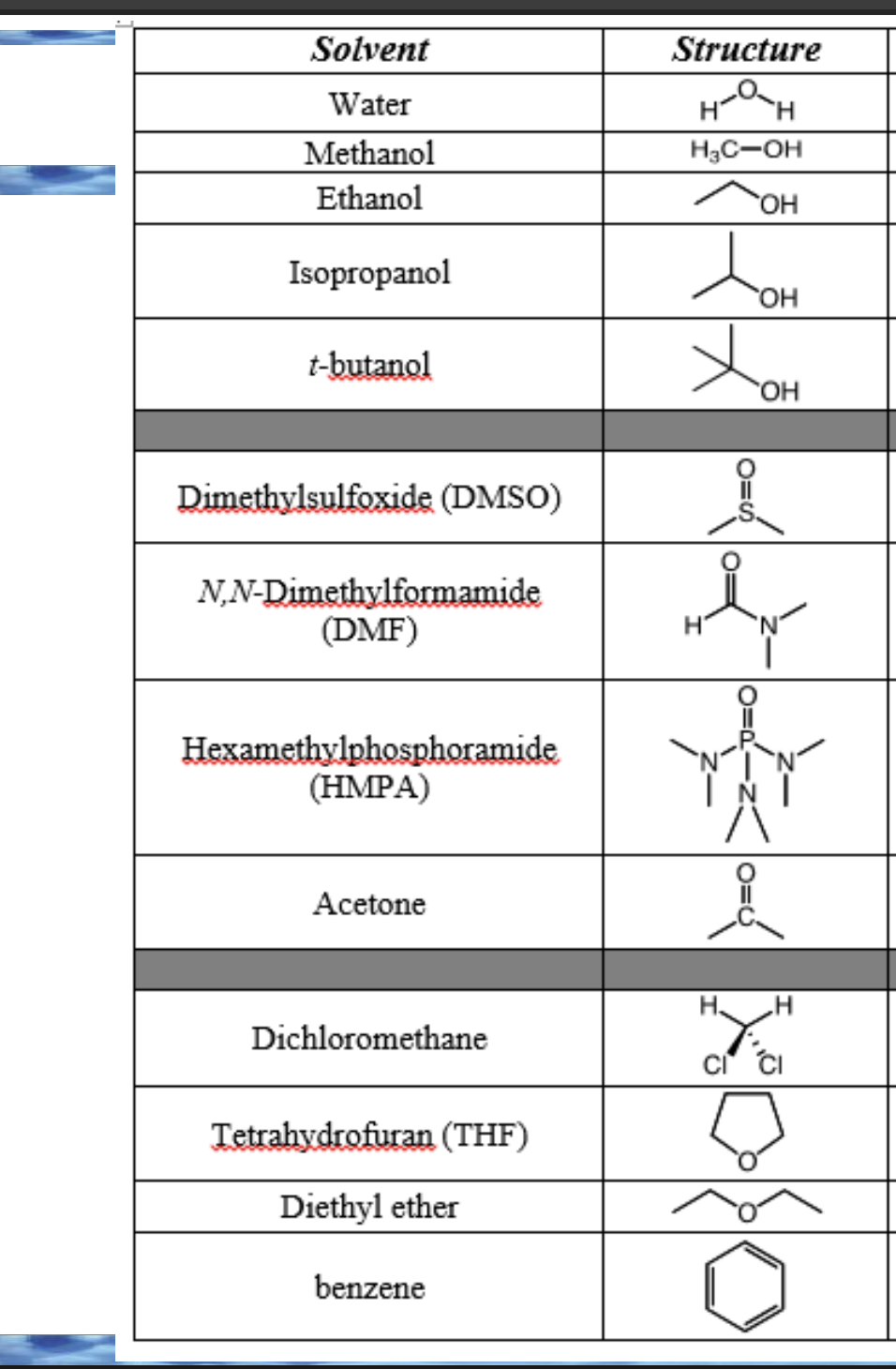
classify these
Front: What is nucleophilicity?
Back: A kinetic property measuring how fast a nucleophile attacks carbon.
Front: What is basicity?
Back: A thermodynamic property measuring how strongly a base binds H⁺.
Front: Which property is measured by reaction rate?
Back: Nucleophilicity.
Front: Which property is measured by equilibrium position?
Basicity
Front: What does a lower activation barrier mean for nucleophilicity?
Back: Faster reaction → stronger nucleophile.
Front: In protic solvent, are larger or smaller nucleophiles better?
Back: Larger.
Front: Rank halides by nucleophilicity in protic solvent.
Back: I⁻ > Br⁻ > Cl⁻ > F⁻.
Front: Are neutral molecules good nucleophiles in protic solvent?
Back: No.
Front: Within a period, which direction increases nucleophilicity?
Right to L
Across a period, nucleophilicity follows basicity.
1⃣ Atoms get less electronegative
They hold electrons less tightly
Lone pairs are more available to attack
Example:
O is more electronegative than N
N holds electrons less tightly → better attacker
Front: Why does nucleophilicity increase right to left in a row?
Back: Electrons are held less tightly.
Front: Which is the best nucleophile in this set: F⁻, OH⁻, NH₂⁻, CH₃⁻?
Back: CH₃⁻.
Which is the best nucleophile?
F⁻, OH⁻, NH₂⁻, CH₃⁻
All of these are:
Negatively charged
Same period (row 2)
That tells you exactly which rule to use.
Front: Given the same nucleophilic atom, which is more nucleophilic: neutral or anion?
Anion
Front: When the nucleophilic atom is the same, what controls nucleophilicity?
Back: Basicity.
Front: Rank these by nucleophilicity: RCOO⁻, HO⁻, RO⁻
Back: RCOO⁻ < HO⁻ < RO⁻
Acetate is a:
Good leaving group
Weak base
But:
Good leaving group ≠ good nucleophile
Resonance makes things stable → stable things don’t attack.
Front: How does conjugate-acid pKa relate to nucleophilicity?
Back: Higher pKa → stronger base → better nucleophile.
Front: For neutral nucleophiles, how does atomic size affect nucleophilicity?
Back: Larger atom = better nucleophile.
Front: How does bulkiness affect nucleophilicity?
Back: More bulk = lower nucleophilicity.
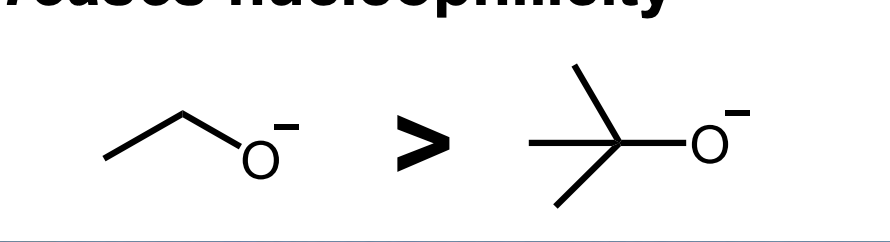
Front: Which is a better nucleophile: ethoxide or tert-butoxide?
Back: Ethoxide.
Q: Best halide nucleophile in polar protic solvent?
A: I⁻
Q: Best halide nucleophile in polar aprotic solvent?
F^-1
Q: Why is F⁻ weak in protic solvents?
A: Strong hydrogen-bonding → heavy solvation
Q: Why is F⁻ strong in aprotic solvents?
A: Poorly solvated + high basicity
Q: One rule to remember solvent effects?
A: Protic → size matters; aprotic → basicity matters
Why the drawings get “bigger” going down a row?
As X gets larger (F → Cl → Br → I):
X orbitals are bigger
σ and σ* orbitals become more polarized
More electron density shifts toward X
Which orbital stabilizes a bond: σ or σ*?
σ stabilizes, σ* destabilizes.