Bio HL1 Test 2 - Kim
1/116
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
117 Terms
carbon can form up to ______ covalent bonds
four
carbon can single, double, and triple bond with
carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus (6 most common elements in life)
carbon forms tetrahedrons with 4 single bonds, and form _______ chains and single or multiple ______.
branched or unbranched; rings
anabolism
joining of a monomer to form a more complex molecule (polymer)
anabolism is done by which reaction
condensation reaction; removal of H2O—removal of -OH (hydroxyl) from one and -H from another; it is endergonic, meaning it requires energy
catabolism
breaking of polymers into monomers—done by hydrolysis, addition of H2O, releases energy
carbohydrate monomer
monosaccharide—typically glucose
monosaccharides are linked by condensation reactions to form
disaccharides and polysaccharides
bond between carb monomers
glycosidic linkage (C-O-C covalent bond)
glucose exists in two forms:
linear and ring
pentose contains carbons, hexose contains _
5 (deoxy/ribose); 6 (glucose)
glucose as energy storage
highly soluble, easily transported in mediums like blood, chemically stable, circulates without breaking down, yields large amnts of ATP when oxidized
polysaccharide
large carb molecules formed by linking many monosaccharides (1,000)
starch
short term plant energy storagge; e.g. amylose—unbranched, helical, preferred form (1-4 bonds); amylopectin—branched (1-4, 1-6 bonds)
1-4 linkages vs 1-6 linkages
linear vs branched
glycogen
short term energy storage in animals; unbranched (1-4 linkages) and branched (1-6 linkages)—found in animal liver, similar to amylopectin but more branched
polysaccharides as energy storage
compact; coiling of chains and branching during polymerization makes it compact, less soluble than glucose due to size—doesn’t interfere with osmotic gradient of cell, easy to add and remove alpha-glucose monomers via condensation and hydrolysis
cellulose
polysaccharide made of beta-glucose, forms plant cell walls, unbranched (1-4 bonds), indigestible for most—fiber—ruminants (cows) can due to bacteria in their specialized stomachs
alpha vs. beta glucose
structural isomers; position of hydroxyl (-OH) on C1 is different—for condensation rxn to occur between two B-glucoses, newly added one must be rotated 180 degrees
alternating beta-glucoses produce
a straight chain, aligning parallel to one another with the ability to group in bundles—regularly spaced -OH groups allow for additional H-bonds to form between adjacent strands—allows for structural support to resist osmotic pressures
lipids
nonpolar molecules that include fats, oils, waxes, steroids; hydrophobic, do not consist of monomers/polymers
triglycerides
condensation joins one glycerol and three fatty acids— -OH (hydroxyl) of glycerol and -COOH (carboxyl) form ester bonds: O-C=O
this produces 3 H2O
phospholipids
joining of a glycerol to one phosphate group and two fatty acids—amphipathic; hydrophilic (water-loving) and lipophilic (fat-loving)
fatty acids
long hydrocarbons that have -COOH groups on one end—varying in number of carbons and double bonds
saturated fatty acids
no double bonds, linear, coming from animal fats (butter, lard), high melting point—solid at room temp.
unsaturated fatty acids
contains double bonds (mono/polyunsaturated), bent, comes from plant oils (olive oil), low melting point—typically liquid at room temp., as double bonds increase, melting point decreases
cis unsaturated fatty acids vs trans unsaturated fatty acids
cis—2 H atoms adjacent to double bonds are on the same side, hydrogens repel one another—liquid at room temp
trans—2 H atoms adjacent to double bond are on different sides, produced through industrial process hydrogenation (banned), makes fats spreadable (margarine), linear and solid at room temp despite double bonds
endotherms store energy with
saturated fats for compact long-term storage
plants store energy with
unsaturated oils that remain fluid for transport and enzyme function
triglycerides—long-term energy storage’s function
carry twice as much energy as carbohydrates, stored in adipose tissue (specialized connective tissue), immiscible with H2O, allowing for compact storage w/o affecting osmotic gradient
triglycerides—thermal insulation’s function
adipose tissue found under skin, reduces heat loss, low thermal conductivity slows heat transfer from body to ENV, important in arctic life (blubber in marine mammals)
amino acids (monomers) build…
polypeptides
amino acid structural components
alpha-carbon—central carbon where all others are attached
-NH2 (amine group)
-COOH (carboxyl group)
hydrogen
R-group (sidem chain)
amino acids are amphiprotic, meaning
-COOH can donate H+ (acid), and -NH2 can accept H+ (base)
R-groups determine
chemical properties and behavior of AAs
R-groups are mainly divided into
hydrophilic or hydrophobic—of hydrophilic they are polar, with some having charges (behaving as acids or bases)—this causes polypeptides to fold and function based on R-group’s position in chain
synthesis of polypeptides
condensation reaction joins two AAs, -NH2 of one AA bonds with another’s -COOH, forming a peptide bond (C-N) (produces 1 H2O), and is catalyzed by ribosomes
polypeptides have a direction; the reaction that takes place is
NH2 losing an H and COOH losing an OH to form H2O, N-terminus is NH2 end and C-terminus is COOH end; repeated sequences of N-C-C-N-C-C creates backbone
essential versus non-essential AAs
essential cannot be made by the body and must be consumed through food—insufficient intake can limit protein production and affect growth, repair, and enzyme function
animal-based foods have similar AA balances as that of humans
non-essential AAs can be made from other AAs or nitrogen-containing compounds
genetic code specifically codes for __ AAs
20
proteome
total amount of protein synthesized in an organism—every organism’s DNA is unique—so is its proteome
polypeptides can contain any # of AAs, arranged in
any order—meaning there are 20^n possibilies (n=# in chain)
examples of polypeptides
insulin—regulates blood glucose levels
hemoglobin—transports oxygen
titin—elastic protein found in striated muscle
primary structure of protein
specific sequence of AAs in a polypeptide—determined by genetic code within DNA, composed of only peptide bonds
precise position of AAs in primary structure determines
3-D shape and function of proteins—structure is precise, predictable, and repeatable despite their complexity
example of one wrong AA
sickle cell anemia
secondary structure of proteins
local folding of polypeptide into shapes, stabilized by H-bonds; C=O and N-H are polar
alpha-helices
a spiral arrangement with H-bonds every 4th AA
beta-pleated sheets
sheet-like arrangement with H-bonds between parallel or antiparallel strands
tertiary structure of proteins and its bonds
specific 3D conformation of a polypeptide—determined by affinity or repulsion between R-groups
bonds: disulfide covalent (S-S)—between two cysteine AAs (strongest, provides stability)
ionic—COOH can donate H+ and NH2 can accept them, becoming negatively and positively charged respectively
hydrogen bonds
hydrophobic interactions—found between nonpolar groups that cluster together away from H2O
quatenary structure of proteins and its bonds
arrangement and interaction of multiple polypeptides
conjugated proteins
contain one or more non-proteins (prosthetic groups); e.g. hemoglobin—4 polypeptides, each with an iron-containing heme group
non-conjugated proteins
composed only of polypeptides; e.g. insulin—2 polypeptides, linked by disulfide bonds; collagen—3 polypeptides, wound together to form a triple helix
proteins’ specific 3D structures determine
their biological functions:
specific DNA sequence → specific AA sequence → unique R-group interaction → 3D shape → function—STRUCTURE DETERMINES FUNCTION
fibrous proteins and example
long, narrow insoluble proteins that have structural roles, e.g. collagen (3 polypeptides in a triple helix)—contains repeating sequences of 3 AAs, one of which preventing alpha-helices from forming, provides high tensile strength (skin, tendons, eyes) to prevent tissue tearing under stress.
globular proteins and example
compact, spherical water-soluble proteins that have metabolic roles, e.g. insulin—has a specific shape that allows it to bind to its receptor (like substrate and enzyme’s active site)—allows specific and unambiguous signals to be sent, like when blood sugar is too high
all living organisms have which genetic material
DNA
DNA stores
hereditary (genetic) information
DNA is made of individual
nucleotides
DNA is passed on from
cell to cell and parent to offspring
some viruses carry ___ as their genetic material
RNA, but this doesn’t negate that all living things have DNA since viruses are nonliving since they can’t reproduce
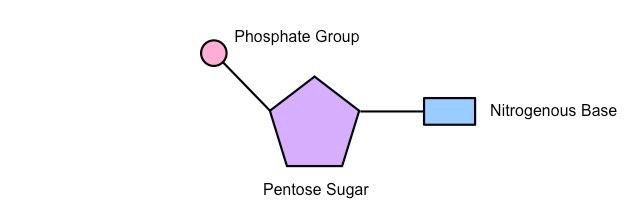
nucleotides components for drawing
5-carbon pentose sugar (deoxyribose or ribose), nitrogenous base attached to 1’ carbon, phosphate group
sugar-phosphate backbone
forms covalent bond between phosphate of one nucleotide and pentose sugar of next nucleotide—called phosphodiester bond—always added in same direction, chain of repeating C,O, and P—alternating sugar and phosphate
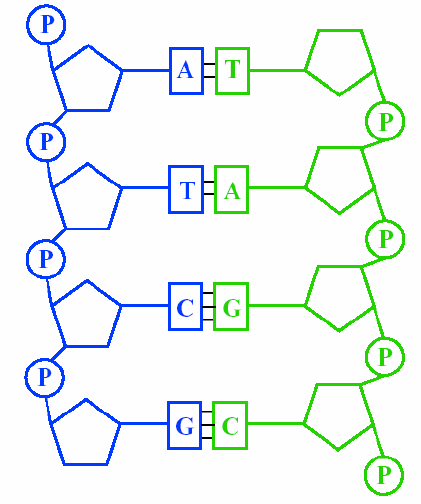
all DNA and RNA bases contain which element
nitrogen
any two bases can be
linked covalently, creating infinite possibilities
purine
A and G, two rings
pyrimidine
C and T, one ring
each base pair contains
one purine and one pyrimidine, equal in width and length, allows DNA to be stable and have variety in sequence
DNA drawing
two strands of nucleotides joined at bases via H-bonds, pairs of complimentary bases
DNA in antiparallel form
two DNA strands run parallel in opposite directions—one strand runs 5’—3’, and one runs 3’—5’
double helix
results in a structure that looks like a twisted ladder, consistent diameter of 2nm—very efficient
semi-conservative replication
each strand serves as a template—added bases are complimentary to bases on template—results in DNA consisting of one original (parent) and one new (daughter) strand
nucleosome
DNA wrapped ~ twice around 8 histone proteins (octamer)—two copies of four different histones form disc-shape
H1 histone
linker histone that assists in bonding of DNA to octamer—all plant and animal DNA have nucleosomes—bacterial DNA doesn’t
DNA vs RNA: strands, sugar type, nitrogenous bases
2 vs 1; deoxyribose vs ribose; AGCT vs AGCU
RNA
single stranded polymer of nucleotides (monomer)—always linked by condensation reaction—removal of H2O— -OH from phosphate and -H from sugar
-O on sugar forms phosphodiester bond
DNA is read
with codons—64 diff. triplet bases—considered to be universal—transferable between species—one codon codes for one AA—AUG codes for start, UAA, UGA, UAG signal stop
at 3’ end, ___ is unbound
at 5’ end, ___ is unbound
pentose sugar; phosphate
tetranucleotide hypothesis
DNA was thought to have consisted of four repeating bases in equal numbers, which wouldn’t account for genetic difference—this is why the 20 AAs to be the genetic material
plant storage / structure
starch-alpha; cellulose-beta
animal storage / structure
glycogen-alpha; chitin-modified/beta
cell membrane and its properties
bilayer of phospholipids that forms a continuous battier, separating the cell from its environment and controls passage of its particles
phospholipid structure
amphipathic, both hydrophilic and lipophilic, polar head consisting of glycerol and phosphate, two nonpolar taios composed of fatty acids, in aqueous environments spontaneously arrange into a bilayer, hydrophobic tails attracted to each other, hydrophilic heads H-bond with cytosolic and extracellular fluids
fluid-mosaic model
phospholipid bilayer with embedded proteins
fluid: phospholipids are free and move laterally
mosaic: different types of proteins (like tiles in a mosaic)
phospholipid bilayer is sustained by hydrophobic interactions, so they drift ______, but not _______.
laterally; transversely
saturated fatty acid structure
straight, packed tightly, increased density and decreased fluidity and permeability for diffusion
unsaturated fatty acid structure
bent, packed loosely, decreased density and increased fluidity and permeability
ratio of saturated and unsaturated fatty acids must be regulated because the membranes must be:
fluid but intact, permeable but not perforated
arctic animals have a ______ proportion of unsaturated fatty acids than saturated
greater
steroid
four fused carbon rings, 3 cyclohexanic rings, 1 cyclopentane ring, 17 carbons total
Cholesterol’s role/location in animal cell membrane fluidity
maintains membrane fluidity and stability in animal cells, steroid rings aligning with fatty acid tails, hydroxyl groups aligning with phosphate heads; commonly located btwn saturated fatty acids
cholesterol properties
controls membrane fluidity by seperating phospholipid tails, too fluid (increased kinetic energy) means increased permeability and increased diffusion, buffers against temperature changes, in high temperatures maintains impermeability and in low temperatures stops crystallization, anchors peripheral proteins
glycoproteins
involved in cell-to-cell recognition, proteins covalently bonded to short, branched monosaccharides, e.g. ABO blood groups
glycolipids
lipid’s polar heads bound to carbohydrate chains, e.g. antigens and antibodies
carbohydrate chains project to the extracellular side, forming
glycocalyx
glycocalyx
carb-rich layer providing protection and allowingg for communication btwn cells
glycoproteins and glycolipid function in cell adhesion
glycoproteins: help cells bind to each other or to ECM
glycolipids: promote membrane stability
glycoproteins and glycolipid function in cell recognition
carb chains serve as identification markers used by immune and signalling systems, seperates “self” from “non-self” cells
cell-adhesion molecules
specialized proteins on cell surface that enables cells to recognize, bind, and communicate with other cells, binds to complimentary molecules on adjacent cells or components of the ECM, allowing cells to organize into tissue, coordinates communication and function, prevents cells from detaching under mechanical stress
different types of CAMs create
junctions (tight, gap)