2.2.12 the Arrhenius equation
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
what happens to the proportion of molecules that have energy equal to or greater than Ea as temperature increasses
greater proporition: increases
what is the relationship between the rate constant, rate of reaction and the fraction of molecules with energy equal or greater than the activation energy
directly proportional
what is the arrhenius equation
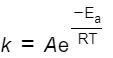
what is k
the rate constant
what is A
the Arrhenius factor
what does A take into account
the frequency of the colliosns with proper orientations
what is Ea and units
activation energy in J mol-1
what is R and units
the gas constant (8.31 J k-1 mol-1)
what is T and what is it measured in
Temperature, K
what is e
mathematical constant
what is the Arrhenius equation using natural logs
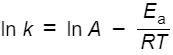
what does an increases in the activation energy mean for the rate of reaction
an increase in Ea means the proportion of molecules which posses the activation energy or greater is lower so rate of reaction and k decreases
in a graph of 1/T against lnk, what is the gradient
-Ea/R
what is the y intercept
lnA
write this as an equation using the formula y=mx+c
lnk = -Ea/R x 1/T + lnA
how can the activation energy be calculated
- gradient x R