Biochem Lec 27- Oxidative Phosphorylation I
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
What does oxidative phosphorylation do? How much ATP is needed everyday?
Oxidative phosphorylation→Production of ATP from the re-oxidation of FADH2 and NADH
Electrons are transferred from NADH and FADH2 to O2 through a series of electron carriers.
We need about 83 kg (!!!!) of ATP everyday to satisfy energy needs. OxPhos efficiently recycles ADP back to ATP.
What is the general flow of oxidative phosphorylation?
Redox power→ Electrochemical gradient→ Chemical energy
Electron transport: Harvest reducing power from NADH and FADH2
Proton gradient: “proton motive force”
ATP synthesis
Describe the parts of a redox reaction using the following reaction: AH2 + B→ A + BH2
AH2 is more reduced than A (more electrons/hydrogens)
B is more oxidized than BH2
In this reaction 2 H and 2 e- are transferred from AH2 to B
AH2 is the reductant/reducing agent
B is the oxidant/oxidizing agent
The product A is oxidized (lost electrons)
BH2 is reduced (gained electrons)
Why is a redox reaction separated into ½ reaction pairs? On what side of the arrow is the reductant/oxidant drawn?
½ reactions→ to judge the power of each oxidant
Oxidant drawn on left side and reductant drawn on right side
What does Eº‘ measure?
It is the biological standard reduction potential→ is a measure of the tendency of a compound to give up or accept electrons under standard biological conditions
Compounds that are strong reductants/high tendency to give up electrons→ Negative Eº‘
Compounds that are strong oxidants/high tendency to accept electrons→ Positive Eº‘
What is the reaction catalyzed by lactate dehydrogenase? What are the half reactions? Is pyruvate or NAD+ a stronger reductant?
NADH + H+ + pyruvate→ NAD+ + lactate
NAD+ + 2e- + 2H+→ NADH + H+: Eº‘= -0.32 volts
Pyruvate + 2e- +2H+→ Lactate: Eº‘= -0.19 volts
NAD+ is a stronger reductant
What is ΔEo’ and how is it calculated? What is ΔEo’ for the lactate dehydrogenase reaction (ΔEo’pyruvate= -0.19V and ΔEo’NAD= -0.32 V)?
ΔEo’ is the change in standard reduction potential for an oxidation-reduction reaction and is a measure of “reducing power”
ΔEo’= ΔEo’oxidant - ΔEo’reductant
ΔEo’= ΔEo’pyruvate - ΔEo’NAD= -0.19 V - (-0.32V)= +0.13 V
How can ΔGo’ be calculated from ΔEo’? What is this value for the lactate dehydrogenase reaction? (ΔEo’= +0.13 V, F= 96.5 kJ/mol, n= number of electrons transferred)
ΔGo’ can be calculated from ΔEo’ using the Nernst equation:
ΔGo’= -nFΔEo’
n= number of e- transferred
F= Faraday’s constant→ 96.5 kJ/mol V
ΔGo’= -nFΔEo’= -(2)(96.5 kJ/mol V)(+0.13 V)= -25.1 kJ/mol
Each reductant and oxidant pair has its own ΔEo’ value→ from these the free energy available from any redox reaction can be calculated
Calculate ΔEo’ for both the NADH and FADH2 reactions.
NADH + H + ½ O2→ NAD+ + H2O
NAD+ + 2e- + 2H+→ NADH + H+: Eo’NAD= -0.32 volts
½ O2 + 2e- + 2H+→ H2O: Eo’oxygen= +0.82 volts
ΔEo’= Eo’oxygen- Eo’NAD= +0.82 V - (-0.32 V)= +1.14 V
FADH2 + ½ O2→ FAD + H2O
FAD + 2e- + 2H+→ FADH2: Eo’FAD= -0.22 V
½ O2 + 2e- + 2H+→ H2O: Eo’oxygen= +0.82 V
ΔEo’= Eo’oxygen- Eo’FAD= +0.82 V - (-0.22 V)= +1.04 V
Calculate ΔGo’ for NADH (ΔEo’= +1.14 V) and FADH2 (ΔEo’= +1.04 V).
NADH:
ΔGo’= -(2)(96.5 kJ/mol V)(1.14 V)= -220.02 kJ/mol
FADH2:
ΔGo’= -(2)(96.5 kJ/mol V)(1.04 V)= -200.72 kJ/mol
What is the cost of making one ATP? How does this relate to the previously calculated free energies of the NADH and FADH2 reactions?
The cost of making one ATP is +30.5 kJ/mol
Theoretically, many ATP should be able to be produced from this reducing power
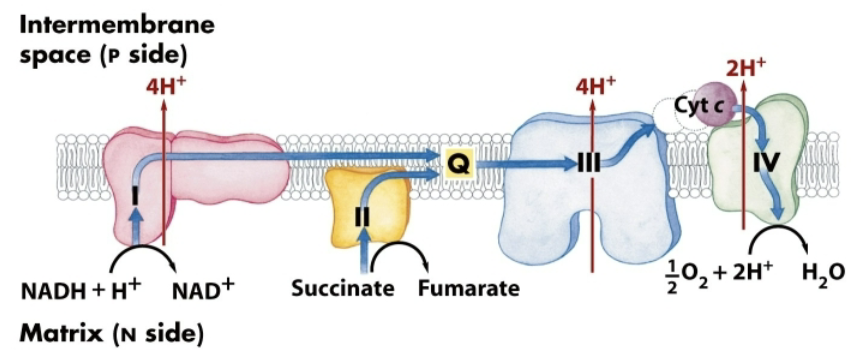
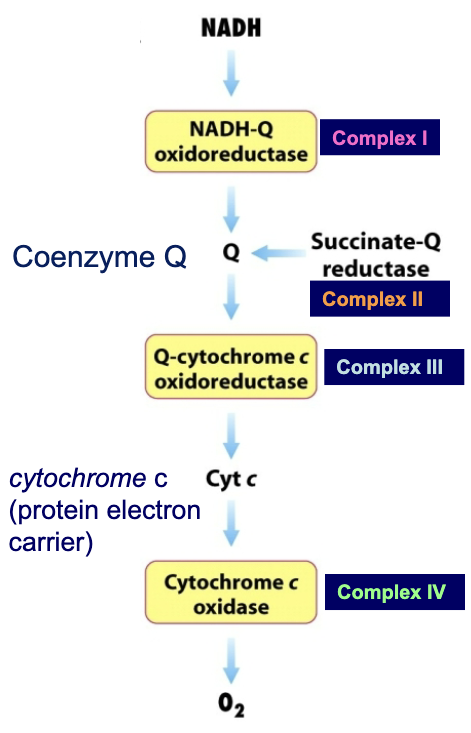
How many large protein complexes make up the electron transport chain?
4:
Complex I→ NADH-Q oxidoreductase
Complex II→ succinate Q-reductase
Complex III→ Q-cytochrome c oxidoreductase
Complex IV→ Cytochrome c oxidase
How do the complexes use NADH and what specific complexes are involved?
Electrons flow from NADH to O2 through four large protein complexes embedded in the inner mitochondrial membrane
Three complexes move protons out of the mitochondrial matrix, generating a proton gradient:
Complex I→ NADH-Q oxidoreductase
Complex III→ Q-cytochrome c oxidoreductase
Complex IV→ Cytochrome c oxidase
What is the role of complex II in the ETC?
Complex II (succinate Q-reductase)→ delivers electrons from FADH2 to complex III
succinate Q-reductase is NOT a proton pump
What are the mobile carriers in the transport chain?
Ubiquinone (coenzyme Q)
Cytochrome C
They connect the transport chains.
What are the electrons donated by NADH and FADH2 passed to? Where are they located?
They are passed to electron carriers:
Coenzyme Q, which is derived from isoprene, binds protons (QH2) as well as electrons and can exist in several oxidation states
Oxidized and reduced Q are present in the inner mitochondrial membrane in what is called the Q pool
Cytochrome C is an electron carrier that employs an iron incorporated into a heme. Cytochrome C carries electrons from Complex III to Complex IV
Overview of ETC
Site of entry for electrons from NADH and FADH2 (succinate) from the CAC and other catabolic pathways
Four multienzyme complexes connected by two mobile carriers: ubiquinone (Q) and cytochrome c
Electrons flow from -Eº’ to + -Eº’
During electron flow, H+ is pumped from the matrix to the cytosolic side of the mitochondrial inner membrane