characterisation of solid state pt 2
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
what are advantages of powder XRD?
(purity, sample prep, changes, equipment)
establishes phase purity
don’t need to grow large single crystals
can monitor structural changes
lab equipment usually sufficient
what does x-ray scattering factors depend on?
what does this mean about pXRD compared to single crystal XRD? 3
depends on number of electrons around atom or ion
light atoms (H, Li) hard to locate
hard to discriminate between atoms with similar numbers of electrons (periodic table/ox states)
heavy atoms dominate diffraction
issues are worse for pXRD
what can you do if no structural model is available?
what is an issue and how can you overcome it
single crystal structure determination is needed
hard to obtain big enough crystals
can use synchrotron radiation
what is the de Broglie relationship
any beam of moving particles will display wave properties according to
λ = h / p
p is momentum

how can you control wavelength of neutrons? give example of technique
what wavelength is needed for neutron diffraction?
by controlling their velocity
e.g. pass fast neutrons through moderators like D2O to slow them
wavelength of ~1Å
is a high or low flux of neutrons needed for diffraction?
what are 2 possible sources
high flux needed
high flux nuclear reactor
spallation sources where neutrons are produced by bombarding metal target with high energy protons (generated by synchrotron)
what in a crystal scatters the neutrons? how is this different from x ray diffraction?
neutrons scattered by atomic nuclei
x rays scattered by electrons
why are bond lengths more accurate from neutron than x ray diffraction?
x ray estimates where the centre of the atom is based on electron density (elliptical electron cloud)
neutron determines location of nuclei
do neutron scattering factors depend on size as with XRD?
how do they change for different atoms/isotopes? light/heavy atoms? Bragg angles?
do not depend on size factors
different for all atoms, and even different isotopes of same atom
relatively similar between light and heavy so light atoms scatter as well as heavy
do not decrease at high Bragg angles
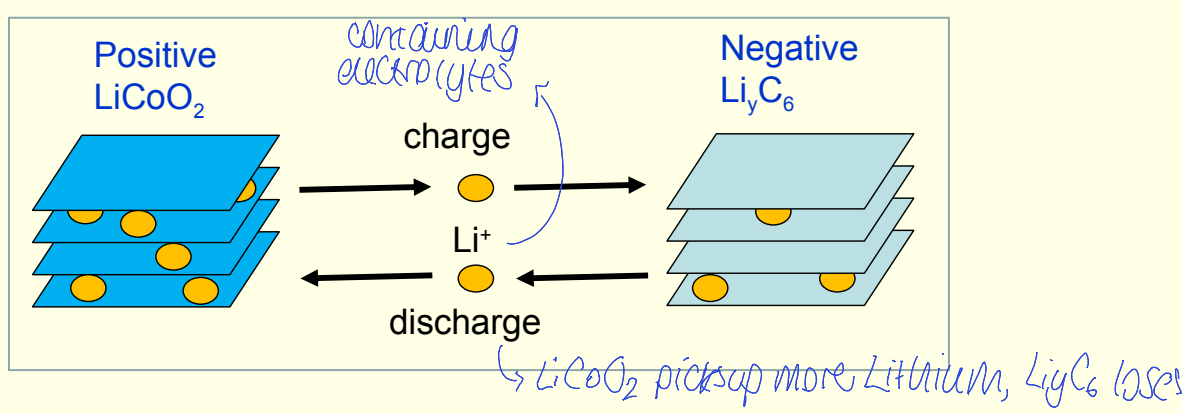
how does charging lithium battery affect structure?
what can pXRD do/not do? what other technique could you use?
causes loss of Li+ from LiCoO2 to give LixCoO2
can monitor whether phase or structural changes are occurring during delithiation
cannot determine x in LixCoO2, could be determined by neutron diffraction
how do neutrons interact with magnetic moment of atom
what is atomic magnetic moment caused by?
neutrons have a spin so have a magnetic moment
can interact with magnetic moment of atom
atomic magnetic moment caused by alignment of electron spins
how can neutron diffraction be used for magnetic moments in structure? what is determined?
magnetic scattering of polarised neutrons from ordered magnetic material
gives magnetic Bragg reflections
can find magnitude and direction of magnetic moments
disadvantages of neutron diffraction 2
expensive
neutrons have low flux - need very large crystals for single crystal studies. longer collection times than X-ray. large banks of detectors often used
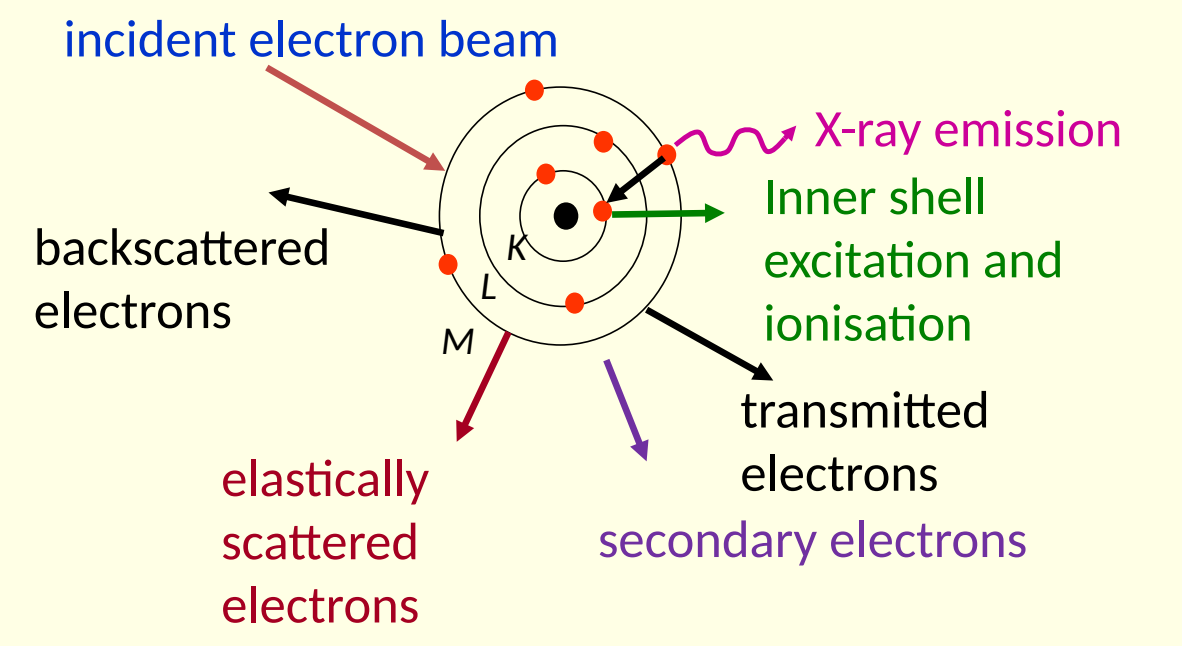
show diagram of atom under bombardment of incident electron beam
how are electrons scattered? what is emitted? show K,L,M
what forms electron diffraction pattern?
what machine is used?
elastically scattered electrons can form diffraction pattern if sample is crystalline
transmission electron microscope used
what do scattering factors depend on for electron diffraction? why
scattering factors depends on atomic number (as with XRD)
interact with both electron density and nuclei
is small or large sample needed for electron diffraction? why?
very high scattering efficiency
only v small sample needed
2 advantages of electron diffraction over XRD/neutron
much more sensitive - only trace amounts of sample needed
can focus on different regions of sample as electron microscope used
2 disadvantages of electron diffraction
restricted to indexing - determining unit cell parameters and miller indices of reflection
full structure solution / refinement is possible but specialist instrumentation is very new and rare
electron diffraction diagram
show L - what is it?
show r - what is it?
show angle
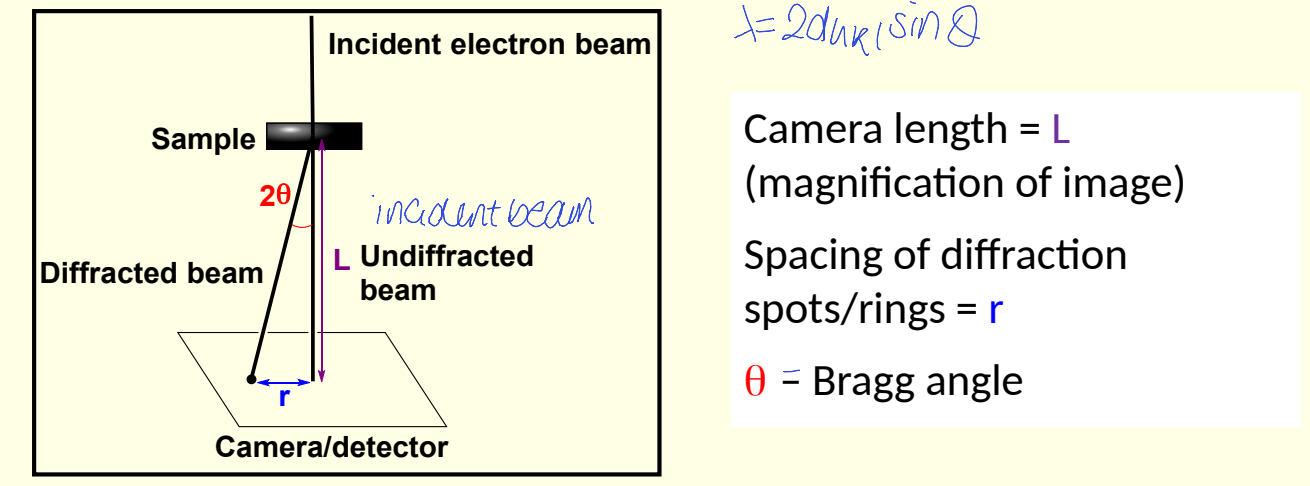
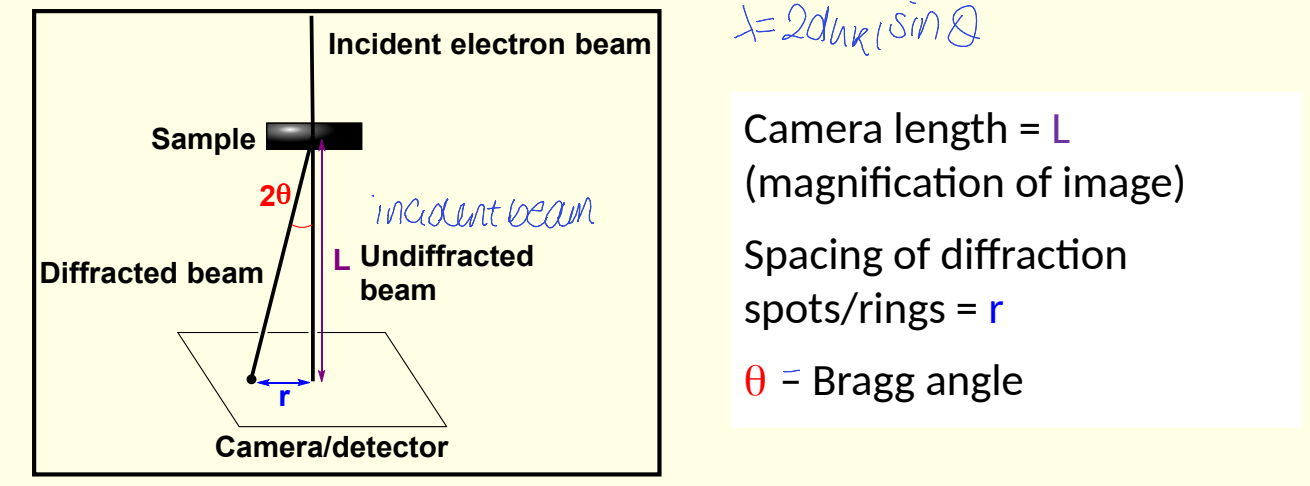
what is the relationship between 2θ, r and L?
what approximation is made?

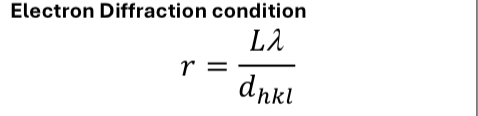
combine 2θ = r/L and Bragg’s law to give electron diffraction equation
what approx is made?

what does wavelength of electrons depend on?
accelerating voltage thus instrument used
what electrons from electron microscopy (bombardment) are used for imaging?
transmitted, secondary and/or backscattered electrons
what does scanning electron microscopy show?
images of surface of sample , with depth
what does transmission electron microscopy show?
how is image formed? what does this mean about the sample? resolution?
image formed by beam of electrons passing through sample so sample must be thin
images are projection through crystal so look 2D
high resolution, down to near atomic scale - shows lattice
what is transmission electron microscopy good for 5
seeing periodic nature of materials (lattice)
composite materials
showing faults in the lattice structure
giving structural information
chemical analysis
what is an issue with transmission electron microscopy?
beam induced damage of samples
can make or break bonds with high energy electron beams
is scanning probe microscopy image formation based on? what is it not based on?
not based on optics
based on the interaction of a sharp probe with the sample surface
how does probe move in scanning probe microscopy?
probe rastered over sample to collect info from many points
scan area in side to side fashion
what must sample be for scanning probe microscopy?
surface must be very very clean to avoid contaminants
what happens in scanning tunnelling microscopy? when does tunnelling current flow and between what?
quantum tunnelling of electrons between the sample and outermost atoms of tip
metal tip brought close enough to sample for overlap of wave functions
tunnelling current flows between tip and surface when voltage applied
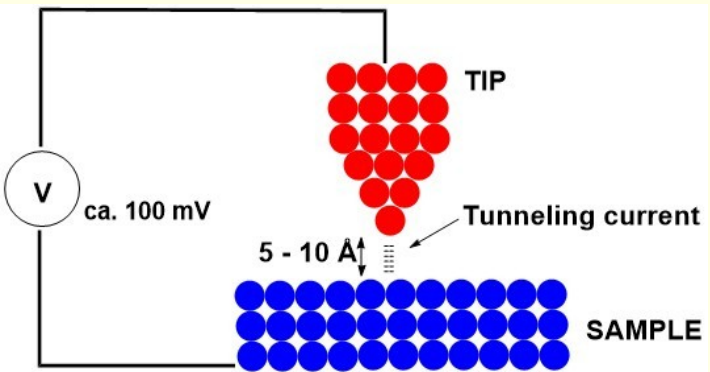
what is tunnelling current sensitive to?
what resolution can be achieved in scanning tunnelling microscopy?
changes in tip surface distance
can achieve atomic resolution as a single atom probes the electron density
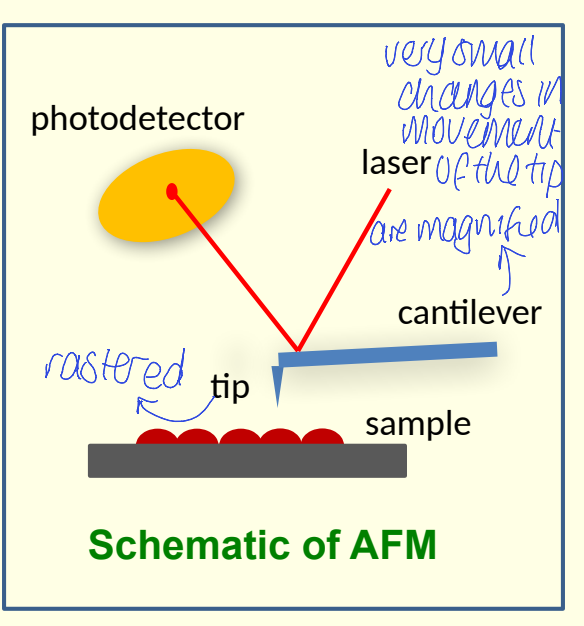
what happens in atomic force microscopy?
where are forces measured? what type of forces? 5
what happens to force while measured? what is used to measure?
measurement of forces between probe tip and sample
could be: van der Waals, frictional, adhesion, magnetic, electrostatic
force is magnified by flexible cantilever. use laser reflected off cantilever to measure
show diagram of atomic force microscopy
show photodetector, laser, sample, cantilever and tip
what is the difference between atomic force microscopy and scanning tunnelling microscopy? (what must sample be like)
STM - must conduct
AFM - can be conducting or non conducting surfaces
what is x-ray emission used for?
what is a negative of it
use characteristic x-ray emission spectra for chemical analysis
doesn’t detect the lightest atoms
what does x-ray absorption give info about? what is used to generate x-rays?
what is it used for/ not used for?
info about local structure and ox states of metals
requires high energy x-rays so synchrotron used
used for metals
lighter atoms (H to B) are difficult / not possible to study
why is X-ray emission characteristic of elements?
different elements have different energies for electronic transitions
X-ray fluorescence
what can it determine? light atoms?
why can it be used for antiquities etc?
elemental composition and stoichiometry
can establish if element is present, compositional ratios between elements
less useful for light atoms as doesn’t detect Li or lighter
not destructive technique - can use on samples where prep is damaging
what is energy dispersive x-ray spectroscopy?
where XRF (X-ray fluorescence) is added to an electron microscope
can study different regions of one sample. electron mapping to give spatial distribution of elements across the sample
show X-ray emission in shells
what are the different types of emission?
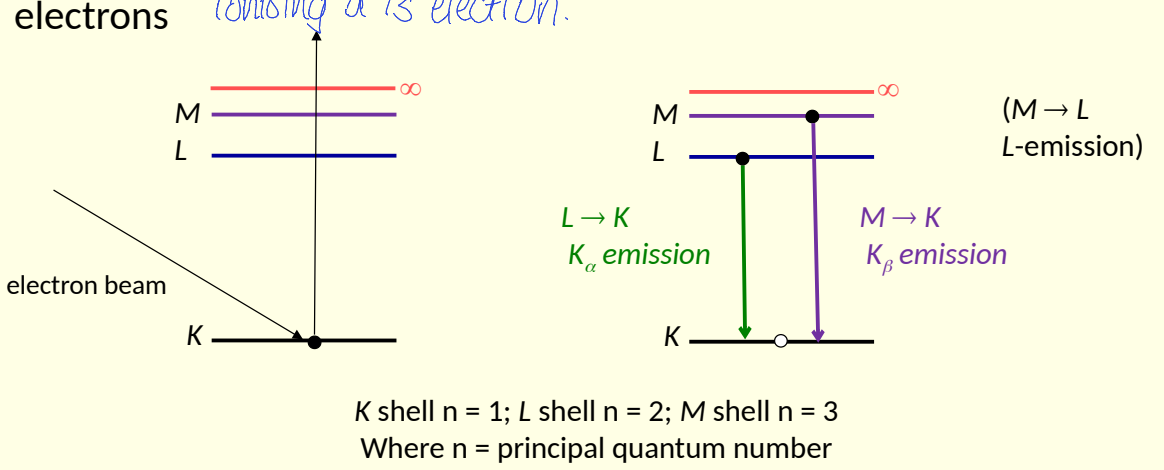
show X-ray absorption in shells
show ionisation and inner shell transitions
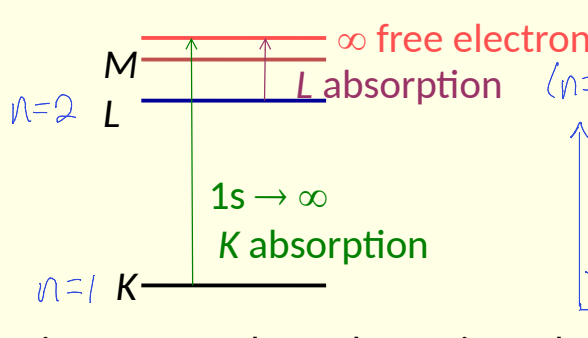
how is X-ray absorption characteristic of an element?
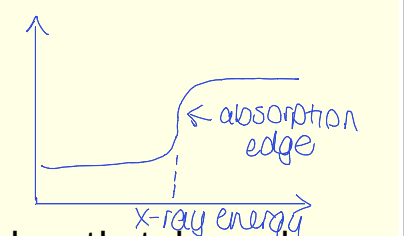
show abs edge
spectra shows absorption edges that depend on relative separation of atomic energy levels
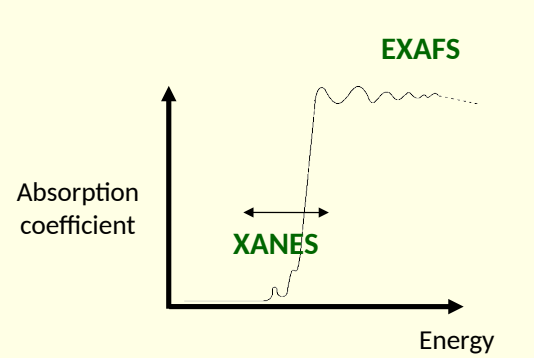
xray absorption near edge structure (XANES) and extended xray absorption fine structure (EXAFS)
what radiation source? how are different energies scanned?
what do they measure? are they good for all elements?
does sample need to be crystalline?
synchrotron radiation with tuneable wavelength to scan diff energies
both measure absorption spectra near absorption edge for one particular element.
usually metal or relatively heavy as light atoms not generally used
sample doesn’t need to be crystalline
what is the difference between XANES and EXAFS?
XANES - absorption around absorption edge
EXAFS - oscillations of absorption coefficient on high energy side of absorption edge
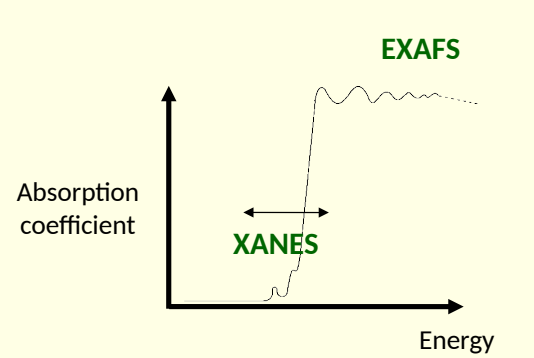
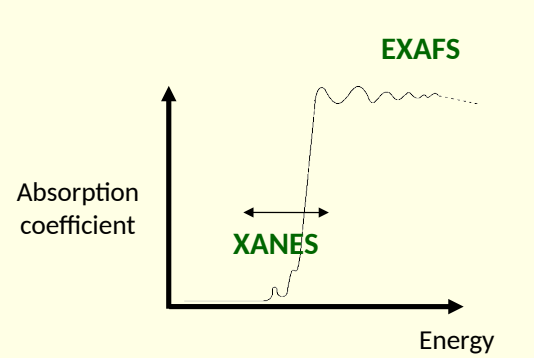
what does XANES peak depend on?3
what does it probe?
ox state
surrounding ligands
nature of bonding
probes local structure around chosen element
why might additional peak be seen?
inter-shell electron transitions
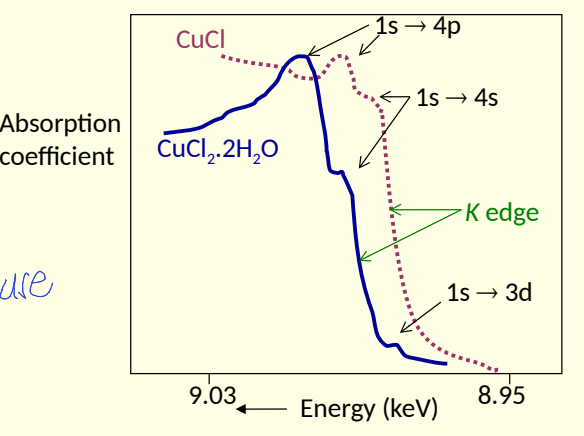
how are XANES studies used with model compounds?
compare obtained spectrum with those of model compounds with known metal oxidation states and coordination environment
what does XANES not give info about?
no info about geometrical structural info e.g. bond length
what does EXAFS study?
oscillations in absorption of xrays for particular metallic element
what are EXAFS measured oscillations caused by?
caused by interference from neighbouring atoms
incident X-ray ionises core electron
ejected photo electron wave scattered by neighbouring atoms
interference between outgoing electron and back scattered ones leads to oscillations
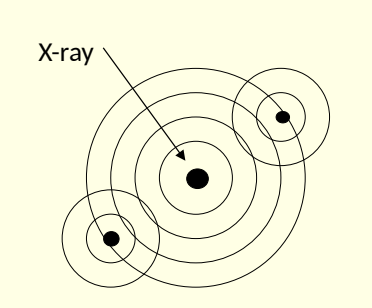
what do oscillations of absorption coefficient depend on?
spatial relationship between absorbing atoms and its neighbours
what transformation of EXAFS gives info about atoms causing interference?
Fourier transformation
shows peaks of distance of coordination spheres
intensity shows what elements there are and how many
what information does EXAFS give and how accurate?
what can therefore be/not be determined?
atomic number of neighbouring atoms to ±2
number of neighbouring atoms to ±1
distance from absorbing atom (bond length) to ±0.01Å, to max ~4Å
bond lengths can be determined but not bond angles
can EXAFS be used for amorphous structures?
yes e.g. powders, glasses, gels
can give bond lengths for complicated non crystalline materials or microcrystalline materials where no good structural model exists