Microbial trophisms and solute transport
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
93 Terms
What are nutrients?
supply of (precursors of) monomers required by cells for growth
What are macronutrients?
Nutrients required in large amounts
What are micronutrients?
Micronutrients - nutrients required in minute amounts
• trace metals and growth factors eg vitamins
• Many microbes can biosynthesise growth factors
How are macronutrients and micronutrients used by microbes?
Microbes convert macro- and micronutrients into cellular components that are essential for growth, maintenance, and function.
Is the macromolecular composition of microbial cells stable?
The macromolecular composition of microbial cells is generally stable but dynamic due to the continuous turnover of macromolecules
How does the macromolecular composition change during storage polymer synthesis?
When storage polymers like lipid globules, glycogen, or polyhydroxyalkanoates (PHA) are synthesised, the macromolecular composition can vary from the normal state.
How can storage molecules in microbial cells be visualised?
Storage molecules such as lipid globules, glycogen, and PHA can often be visualised under a light microscope using phase contrast.
How do microbes acquire energy and nutrients against concentration gradients?
Microbes use active transport mechanisms, often requiring energy input, to move nutrients and energy across cell membranes against concentration gradients, enabling cellular processes to occur.
What is the definition of trophism or tropism?
Trophism or tropism refers to fundamental nutrition involving the actual metabolic exchanges of tissues, typically in the context of how organisms acquire and process nutrients.
How do humans grow in terms of trophism?
Humans grow as obligate aerobically respiring chemoorganotrophs, meaning they require oxygen to metabolise organic compounds for energy.
How can many microbes generate energy?
Many microbes can generate electrochemical gradients across membranes using different sources of energy, allowing them to drive cellular processes.
What types of carbon can microbes use to build cellular components?
Microbes can utilise both organic and inorganic carbon sources to build the cellular components necessary for growth and reproduction.
How do phototrophic organisms capture light energy?
Phototrophic organisms capture light energy using photosensitive receptors connected to a membrane-based electron transport chain (ETC), which converts photons into an electrochemical gradient, typically a proton gradient.
What is the role of the electron transport chain (ETC) in phototrophic organisms?
The ETC in phototrophic organisms turns photons into an electrochemical gradient, often a proton gradient, which is essential for energy production and cellular processes.
What is unique about cyanobacteria’s use of water in their electron transport chain?
Cyanobacteria are unique in using H2O as the terminal electron acceptor in their ETC, which results in the generation of O2 as a byproduct.
How do other microbes differ from cyanobacteria in their electron transport systems?
Other microbes adopt anoxygenic electron transport systems, which do not produce oxygen, using different terminal electron acceptors.
What are examples of photoreceptors involved in phototrophism?
Examples of photoreceptors involved in phototrophism include the Light Harvesting Complex (LHC), Photosystem, and bacteriorhodopsin, which are connected to membrane-based ETCs.
Are there photoreceptors that are not connected to a membrane-based ETC?
Yes, some photoreceptors are not connected to a membrane-based ETC and are involved in regulating gene expression instead.
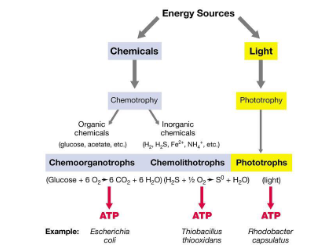
What types of organisms are chemotrophic?
Chemotrophic organisms include both lithotrophs and organotrophs, which obtain energy from chemicals rather than light.
What do lithotrophs do to obtain energy?
Lithotrophs transform chemical energy from reduced inorganic compounds into biological energy by oxidising these compounds, generating high-energy molecules like NADH, NADPH, and FADH2.
How do lithotrophs generate energy through oxidation?
Lithotrophs generate reducing power (e.g., NADH, NADPH, FADH2) by oxidizing reduced inorganic compounds, and they may also generate a chemical gradient, often a proton gradient, across a membrane.
What are some examples of lithotrophic oxidation processes?
Examples of lithotrophic oxidation processes include the oxidation of ammonia, reduced iron (FeII), nitrogen-containing compounds (nitrification), hydrogen, arsenic, and others.
How is lithotrophy often confused with autotrophy?
Lithotrophy is often confused with autotrophy because both processes involve the use of inorganic compounds for energy; however, autotrophs use CO2 as a carbon source, while lithotrophs oxidise inorganic compounds for energy.
What are organotrophs (heterotrophs) in chemotrophic organisms?
Organotrophs (also known as heterotrophs) are chemotrophic organisms that obtain chemical energy exclusively from reduced organic compounds, which they transform into biological energy.
How do organotrophs generate biological energy?
Organotrophs generate biological energy by oxidising reduced organic compounds, producing reducing power (e.g., NADH, NADPH, FADH2) and ATP, which are all high-energy compounds.
What are some examples of organotrophic compounds?
Examples of organotrophic compounds include sugar polymers, fats, proteins, dietary fiber, glucose, pyruvate, and succinate.
What is the key difference between organotrophs and lithotrophs?
Organotrophs (heterotrophs) derive energy from reduced organic compounds, while lithotrophs obtain energy from reduced inorganic compounds.
What determines the type of energy tropism in organisms?
The nature of the source of electrons feeding an electron transport chain (ETC) or generating reduced cofactors determines the type of energy tropism, such as phototrophy, lithotrophy, or organotrophy
What do autotrophic organisms use one-carbon (C1) compounds for?
Autotrophic organisms use one-carbon (C1) compounds to synthesise C-C bonds, generating metabolites and biomacromolecules through a process called carbon fixation or primary production.
Is carbon fixation an energetically cheap or expensive process?
Carbon fixation is an energetically expensive process, as it requires significant energy to generate C-C bonds.
What are some sources of C1 compounds that microbes can fix, other than CO2?
In addition to CO2, microbes can fix other C1 compounds such as CO, cyanide, cyanate, thiocyanate, carbonate, and carbide, showing a diversity in C1 sources
What are the main pathways for microbial autotrophic carbon fixation?
The main pathways for microbial autotrophic carbon fixation include the Calvin–Benson–Bassham (CBB) cycle, Reverse TCA cycle, 3-hydroxypropionate cycle, and the Reductive acetyl-CoA pathway (Wood–Ljungdahl pathway).
What are additional prokaryotic pathways for assimilating one-carbon organic compounds?
Additional prokaryotic pathways for assimilating one-carbon organic compounds include the ribulose monophosphate cycle and the serine pathway.
What does the diversity of autotrophic pathways indicate?
The diversity of autotrophic pathways demonstrates that autotrophy has evolved multiple times on Earth, allowing organisms to adapt to various environmental conditions.
Why is autotrophy important in the context of climate change?
Autotrophy is a major focus for mitigating the climate emergency, as engineering biology and biotechnology may help optimise carbon fixation processes to reduce atmospheric CO2.
What do heterotrophic organisms use to synthesise metabolites and biomacromolecules?
Heterotrophic organisms use one or more organic compounds to synthesise metabolites and biomacromolecules.
What allows heterotrophic microbes to use a wide range of organic compounds?
The wide range of catabolic pathways (metabolic versatility) enables heterotrophic microbes to use most types of organic compounds.
What is the significance of microbial diversity in terminal electron acceptors?
Microbial diversity in terminal electron acceptors allows heterotrophic organisms to adapt to various environments and utilise different compounds for energy production.
What are photoheterotrophs?
Photoheterotrophs are organisms that use light as their energy source and organic compounds as their carbon sources.
What is a chemoorganotroph?
A chemoorganotroph is by definition a heterotroph, as it obtains both its energy and carbon from organic compounds.
What does the term "heterotrophy" refer to?
Heterotrophy refers to the use of organic sources of electrons and carbon for energy and metabolic processes.
What makes mixotrophic organisms the most versatile?
Mixotrophic organisms can use both organic and inorganic sources of carbon, as well as solar and chemical energy sources, allowing them to adapt to a wide range of environments.
How do mixotrophic organisms regulate their metabolic pathways?
Environmental sensing of nutrient and energy availability directs gene expression in mixotrophic organisms, enabling them to switch between different energy and carbon sources.
What are syntrophic organisms?
Syntrophic organisms collaborate to degrade or synthesise substances that neither can do alone, enabling the completion of metabolic pathways that would otherwise be thermodynamically unfavourable.
What is the difference between obligate and facultative organisms?
Obligate organisms have essential metabolic requirements they must fulfill, while facultative organisms are more versatile and can switch between metabolic modes depending on conditions and energy/nutrient availability.
What is the difference between aerobic and anaerobic respiration?
Aerobic respiration uses oxygen as the terminal electron acceptor in the electron transport chain (ETC), while anaerobic respiration uses other terminal electron acceptors in the absence of oxygen.
What is fermentation?
Fermentation is the incomplete oxidation of organic compounds, producing intermediate products as fermentation products. It requires ETC-independent metabolic conversions to balance redox reactions.
How are electron donors and acceptors often confused?
Microbes can use a wide range of electron donors and acceptors, leading to confusion about which molecules are involved in their metabolism.
What factors influence nutrient uptake and solute transport across microbial membranes?
Concentration of the nutrient
Type of compound and its permeability
Barriers to the cytoplasm
Membrane potentials
Chemical gradient (e.g., proton gradient, ΔpH, pmf)
Charge difference (ΔΨ)
Energy requirements
What is the prokaryotic cell envelope and what does it do?
The prokaryotic cell envelope surrounds the cytoplasm and has a different structure in Gram-positive, Gram-negative bacteria, and Archaea.
The cytoplasmic membrane acts as the main barrier for the movement of nutrients and waste products in and out of all prokaryotic cell types.
What are the functions of the cytoplasmic membrane?
Permeability barrier: Polar and charged molecules must be transported, and transport proteins accumulate solutes against the concentration gradient.
Protein anchor: Holds transport proteins in place.
Energy conservation and consumption: Generates membrane potentials, which are important for energy processes.
What are the factors that make transport across a membrane energetically favourable?
Concentration dependence: Solutes move toward equilibrium across the membrane, following the concentration gradient.
Electrochemical dependence: Solutes move toward charge equilibrium across the membrane, following the charge gradient.
Transport occurs when both concentration and charge gradients are involved.
What is simple diffusion in passive transport of solutes?
No energy requirement
Solute moves with the concentration gradient
Dependent on permeability coefficient of the solute
Not a specific process, meaning it does not require transport proteins.
Why do polar solutes need alternative paths to cross the cell membrane?
Energy changes in simple diffusion:
Removal of the hydration shell for hydrophilic solutes is highly endergonic, making the activation energy (∆G‡) for diffusion through the lipid bilayer very high.
In facilitated diffusion:
Transport proteins reduce the ∆G‡ for transmembrane diffusion by forming noncovalent interactions with the dehydrated solute, replacing solute-water hydrogen bonds.
These proteins provide a hydrophilic transmembrane pathway for easier solute passage.
What are ionophores in passive transport?
Ionophores are molecules that facilitate the movement of ions across cell membranes, speeding up ion transmembrane movement in accordance with their electrochemical gradients.
What do ion channels do in passive transport?
Ion channels allow ions to pass through the membrane, speeding up transmembrane movement by following the electrochemical gradient without requiring energy.
How do passive transporters differ from active transporters?
Passive transporters move solutes with their electrochemical gradient without requiring energy, while active transporters move solutes against their gradient and require energy input.
What type of energy is required for active transport?
Active transport requires energy such as ATP, a gradient of a second solute, or a high-energy compound to move solutes against their concentration gradient.
What is the role of active transporters in cells?
Active transporters pump specific solutes against their concentration gradients, ensuring the proper distribution of nutrients and ions across the cell membrane.
What is an example of energy sources used by active transporters?
Active transport can use ATP, a proton motive force (pmf), or a gradient of a second solute to drive the transport process.
What is the difference in solute movement between passive and active transport?
Passive transport moves solutes along their electrochemical gradients (no energy required), while active transport moves solutes against their gradients, requiring energy input.
What does the electrochemical gradient refer to?
The electrochemical gradient refers to the combined effect of concentration gradients and charge differences across the membrane, which drives solute movement in passive transport.
How do transport proteins help in facilitated diffusion?
Transport proteins in facilitated diffusion provide a hydrophilic pathway for solutes to cross the membrane, reducing the energy barrier for the process.
What are the 5 classes of membrane transport systems?
Pores and channels
Transmembrane electron carriers
Electrochemical-potential-driven transporters
Group translocators
Primary active transporters
What are electrochemical-potential-driven transporters also known as?
Electrochemical-potential-driven transporters are also called ‘simple’, ‘passive’, or ‘secondary’ transporters.
What is the function of group translocators?
Group translocators (mostly microbial) phosphorylate the solute being transported, with the phosphorylation provided by phosphoenolpyruvate via a Phosphorelay Transport System (PTS).
What are primary active transporters also called?
Primary active transporters are also called ‘ABC transporters’ (ABC stands for ATP-Binding Cassette).
Do group 3 and 5 transporters modify the solute during transport?
No, group 3 (electrochemical-potential-driven) and group 5 (ABC) transporters do not modify the solute upon transport.
What is the role of transmembrane electron carriers in membrane transport?
Transmembrane electron carriers (not shown in the figure) are involved in transporting electrons across the membrane, typically related to processes like respiration.
How does the Phosphorelay Transport System (PTS) contribute to group translocators?
In group translocators, the Phosphorelay Transport System (PTS) provides the phosphate group (~P) from phosphoenolpyruvate to phosphorylate the solute being transported.
What is the energy source for primary active transporters (ABC transporters)?
Primary active transporters, or ABC transporters, use ATP as the energy source to transport solutes against their concentration gradient.
What type of transport system is involved in pores and channels?
Pores and channels provide passive movement of solutes across the membrane, often by diffusion, and do not require energy.
What distinguishes electrochemical-potential-driven transporters from primary active transporters?
Electrochemical-potential-driven transporters (group 3) use the existing electrochemical gradient to move solutes, while primary active transporters (ABC transporters) directly use ATP to drive solute movement against the gradient.
What is a uniporter in solute translocation?
A uniporter transports a single solute either via mediated diffusion or in a membrane potential-dependent manner (if the solute is charged).
What do symporters do in solute translocation?
Symporters transport ≥2 solutes in the same direction, using the electrochemical potential gradient to drive the reaction.
How do antiporters function in solute translocation?
Antiporters transport ≥2 solutes in opposite directions, using the electrochemical potential gradient to drive the reaction.
What is the role of the electrochemical potential gradient in symporters and antiporters?
The electrochemical potential gradient is used to drive the transport of solutes in both symporters (same direction) and antiporters (opposite directions).
What is an example of a prokaryotic symporter?
An example of a prokaryotic symporter is lac permease, which is driven by proton motive force (pmf). It cotransports lactose (a disaccharide of glucose and galactose) and H+ ions in the same direction, dissipating the pmf.
Why is there a dependency on a Na+ - H+ antiporter for continued Lac activity?
For continued lac permease activity, Na+ - H+ antiporters are needed to maintain the proton motive force (pmf). The antiporter transports H+ out of the cell and Na+ in, maintaining the electrochemical gradient needed for symporter function.
What does the Na+ - H+ antiporter do in prokaryotic cells?
The Na+ - H+ antiporter transports H+ out of the cell and Na+ in the opposite direction. This helps maintain the proton motive force (pmf), essential for processes like symport in the lac permease system.
Why don't humans have disaccharide transport systems like lac permease?
Humans lack disaccharide transport systems and only have monosaccharide transporters. Thus, they do not utilise symporters like lac permease for disaccharides such as lactose.
How do Group 4 translocation systems (PTS) function in bacteria?
Group 4 translocation systems, like the phosphotransfer system (PTS), combine solute uptake and phosphorylation. The substance is chemically modified (phosphorylated) during transport in an ATP-independent manner, with PEP (phosphoenolpyruvate) driving the transport instead of the proton motive force (pmf).
What drives transport in Group 4 translocation systems like PTS?
In Group 4 translocation systems, the transport is driven by the dephosphorylation of PEP (an energy-rich organic compound), not by the proton motive force (pmf).
What are the components of the PTSGlc system in Escherichia coli?
The PTSGlc system in Escherichia coli consists of five proteins:
Enzyme I (EI)
Enzyme IIa (EIIa)
Enzyme IIb (EIIb)
Enzyme IIc (EIIc)
HPr (histidine-containing protein)
How does the phosphorylation relay work in the PTS system?
In the PTS system, the phosphorylation relay occurs in the following sequence:
PEP → EI
EI → HPr
HPr → EIIabc
Finally, EIIc transports and phosphorylates the solute (e.g., glucose).
What is the role of EIIabc in the PTS system?
The EIIabc complex is specific for the particular sugar/solute being imported, such as glucose. It is responsible for the final transport and phosphorylation of the solute.
What is shared between different PTS systems?
The EI and HPr proteins are shared between different PTS systems and are non-specific, meaning they can be used by multiple transport systems for various solutes.
What is the primary function of Group 5 transport systems, specifically ATP-Binding Cassette (ABC) transporters?
ABC transporters are primary active transport systems that use ATP hydrolysis to drive the uptake of organic and inorganic compounds into cells, making the process thermodynamically favorable.
How many different systems of ABC transporters have been identified in prokaryotes?
Over 200 different ABC transport systems have been identified in prokaryotes for the transport of both organic and inorganic compounds.
What is the substrate affinity like in ABC transporters?
ABC transporters exhibit high substrate affinity, allowing them to effectively capture and transport low concentrations of solutes.
What are the three distinct protein domains in an ABC transporter system?
An ABC transporter system consists of three distinct protein domains:
ATP-hydrolysing protein (domain)
Transmembrane protein domain, often containing 12 α-helices interconnected by linker regions
Extracellular binding protein
What is the difference in substrate-binding proteins between Gram-negative and Gram-positive organisms in ABC transporters?
In Gram-negative bacteria, periplasmic binding proteins bind the substrate.
In Gram-positive bacteria and Archaea, substrate-binding proteins are located on the external surface of the cytoplasmic membrane.
What role does ATP hydrolysis play in ABC transporters?
ATP hydrolysis provides the energy required for the active transport of substrates through the ABC transporter, enabling the movement of solutes against their concentration gradient.