Chem Group 17
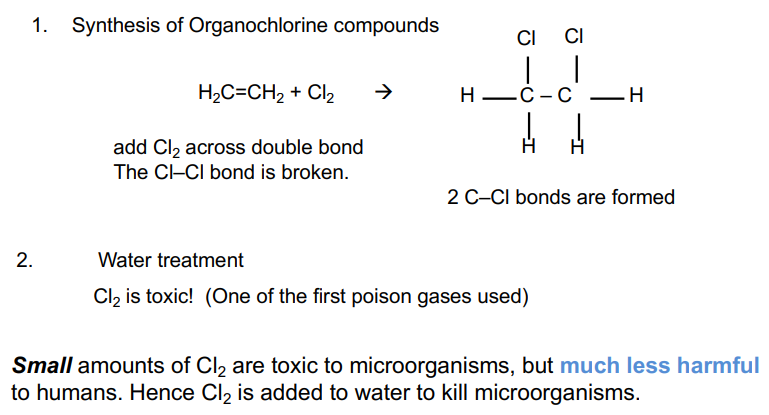
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
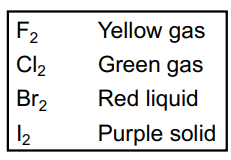
Physical States
Oxidation States
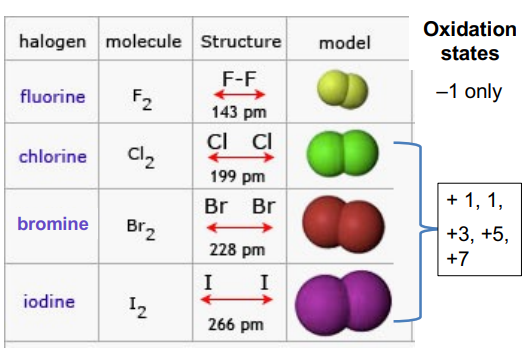
Type
All are nonmetals
Trends down group
include increasing atomic size and decreasing electronegativity. The ionization energy decreases down the group, making it easier for atoms to lose electrons.
Production of Difluorine, F2
Industrially: from fluorspar rock (rich in CaF2)
Step 1. heat with H2SO4 to produce
HF 2 CaF2 + H2SO4 → 2 HF + CaSO4HF
Step 2: reaction of HF with KF to form potassium bifluoride [why? see next step!]
4 HF + KF → KHF2
Step 3: Electrolysis of potassium bifluoride
2 KHF2 → 2 HF + H2 + F2
Laboratory: F2 is rare! Highly dangerous, as is HF
Production of Dichlorine, Cl2
Industrially: Electrolysis of brine (aqueous NaCl)
2 H2O (l) + 2 NaCl (aq) ® H2 (g) + Cl2 (g) + 2 NaOH (aq) (Electrochemical oxidation of Cl– to Cl2)
Co-products are “caustic soda” (sodium hydroxide) and hydrogen gas
Laboratory: (i.e. small scale)
Chemically oxidize Cl– to Cl2 using a good oxidizing agent
O.S. –1 O.S. 0
General approach: oxidize the anions, as above • Br2 and I2 much easier to isolate – easier to oxidize with sulfuric acid • F2 was the last to be isolated
Diatomic halogens X2: Bond lengths & energies
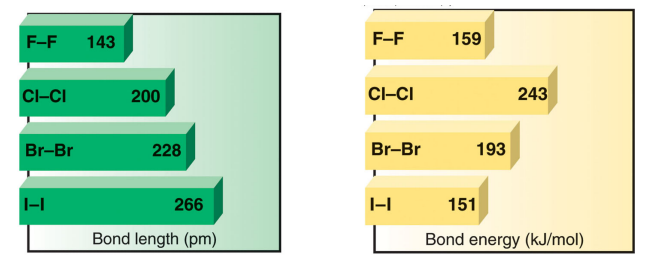
F2: anomaly in the trend down the group
The F–F bond is weaker than expected because the small size of F results in a much shorter bond. Bond energy of F2 is only 8 kJ/mol higher than I2
Short bond à high repulsion between lone pairs on the two F atoms
this underlies the high reactivity of F2
Reactivity of the Halogens, X2
needs only 1 electron to fill its valence shell. The elemental halogens X2 are therefore reactive oxidants
all are electronegative enough to behave as nonmetals (gain e- in chemical reactions: cf. characteristic rx of metals = lose electrons)
The typical reactivity of the halogens decreases down the group, reflecting the decrease in electronegativity. Reactivity of the Halogens, X2. F2 is the most reactive in the series, I2 is the least reactive (despite its bond energy being lowest)
In fact the halogens can engage in several reactions: accept one electron to form a halide anion, accept an electron pair donated by a nonmetal atom, donate an e-pair to transition metals, i.e. function as a Lewis base
H2, water, hydroxide
The halogens (X2) oxidize many metals and nonmetals. The reaction with H2 is characteristic:
X2 + H2(g) → 2 HX(g)
OS 0 0 OS 1+ 1–
The halogens disproportionate in water to HX and the hypohalous acid
X2 + H2O (l) → HX(aq) + HOX(aq) (X = Cl, Br, I)
OS of X: 0 1 - 1+
in basic water, reaction proceeds further to form hypohalite oxyanions (e.g. NaOCl) The halogen is still in the +1 oxidation state
at higher temperatures, halates (OS +5) are formed
Relative oxidizing ability of X2 & reducing ability of X–
A. The oxidizing ability of X2 (ability to abstract an electron) decreases down the group…. and the reducing ability of X– (ability to give up an electron) increases
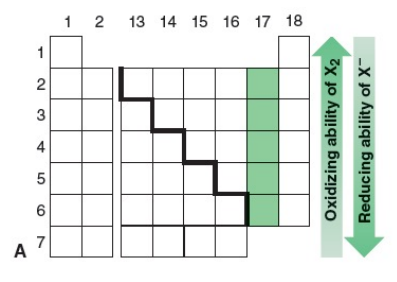
Relative oxidizing ability of X2 & reducing ability of X–
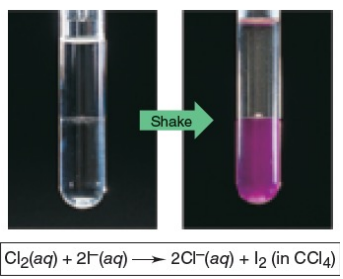
B. Cl2 will therefore oxidize aqueous solutions of iodide salts (I–) to I2 ... ...but the corresponding reaction of I2 does not occur: I2 does not oxidize Cl– ions
Cl2 is a stronger oxidizing agent than I2
Reactivity of Difluorine
most reactive of all the elements
due to weak F–F bond... and strong bond of F with other elements
recall it's only 8 kJ/mol stronger than I2, and it forms stronger bonds
F2 reacts with every element except He, Ne, and Ar
Oxidizing Ability of Difluorine
F2 easily oxidizes other elements
2 Fe (s) + 3 F2 (g) à 2 FeF3 (s)
Iron oxidized from 0 to +3
S (s) + 3 F2 (g) à SF6 (g)
Sulfur oxidized from 0 to +6 (its highest oxidation state)
F2 oxidizes water to O2
Reduction: F2 (g) + 2 e– → 2 F– (aq) Eo = + 2.87 V
Oxidation: 2 H2O (l) → 4 H+ (aq) + O2 (g) + 4 e– Eo = –1.23 V
Overall: 2 F2 (g) + 2 H2O (l) → O2 (g) + 4 HF (g)
In reaction with metals, water is reduced to form H2 Here, water is oxidized by F2 to form O2: much less common
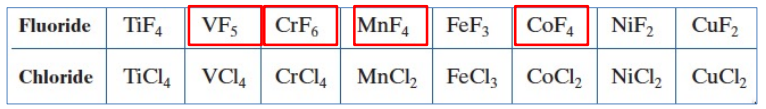
Oxidation States Attainable via F2 vs Cl2
oxidizing power of F2 can often give access to higher oxidation states than seen for the other halogen
Metal Halides:
2 Fe (s) + 3 F2 (g) → 2 FeF3 (s)
2 Fe (s) + 3 Cl2 (g) → 2 FeCl3 (s)
But: across the 1st row transition metals, FOUR have higher OS for F than Cl
Uses of Cl2
Uses of Chlorine: Bleach in Water Treatment
application results from the strong (but not crazy aggressive) oxidizing capabilities of Cl2:
Cl2 (aq) + 2 e– → 2 Cl– (aq) Eo = + 1.36 V (favourable)
F2 (aq) + 2 e– → 2 F– (aq) Eo = + 2.87 V (crazy)
The reaction of Cl2 with water produces “bleach” in a redox process:
0 -1 +1 (Disproportionation)
Cl2 + H2O → HCl (g) + HOCl (aq)
equilibrium lies to the left (Cl2 favoured by 2:1), but: driven by rx with water
HOCl (aq) + H2O (l) → H3O+ (aq) + ClO–
NaOCl = "bleach." Hypochlorite: strong bleaching agent (oxidant)
Household Powdered Bleach
Ca(OCl)2 = one of the active compounds in "bleaching powder"
Mixture of:
• calcium hypochlorite Ca(OCl)2
• dibasic calcium hypochlorite, Ca3(OCl)2(OH)4
• dibasic calcium chloride, Ca3Cl2(OH)4
• relatively stable but >oxidizing than "liquid bleach"
mixing bleach with acidic cleaning agents is highly inadvisable
ClO– (aq) + H+ (aq) → HOCl (aq)
HOCl (aq) + Cl– (aq) + H+ (aq) → Cl2 (g) + H2O (l)
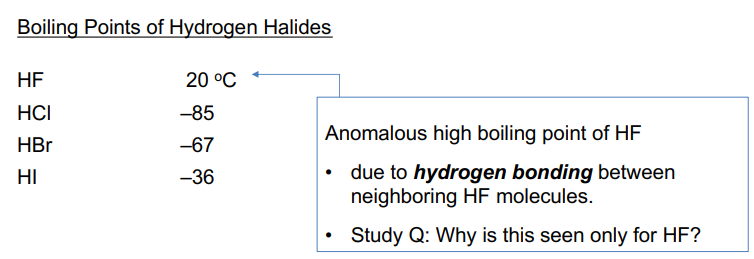
Hydrogen Halides
weaker H-X bond = stronger acid
F-F bond is very weak, but H-F bond is very strong
H is substantially positive if bound to highly electronegative element
• smaller X– = greater attraction for H+ = weaker acid
• larger X = more diffuse charge, H+ less attracted: stronger acid.
• Hence > acidity down group
• Weak acidity of HF does not mean that it is unreactive -- only that it does not ionize completely in water
Hydrogen Fluoride, HF
Extremely poisonous (exposure is deadly)
Very corrosive (even though it is only a weak acid)
One of the few substances that will attack glass
SiO2 (s) + 4 HF (aq) ® SiF4 (aq) + 2 H2O (l)
This reaction is used to etch glass (convert to soluble SiF4)
Hydrogen Chloride, HCl
Highly water–soluble: concentrated HCl = 12 Molar..... Very strong acid
Production:
Method 1: Reaction of NaCl with sulfuric acid:
150oC 2 NaCl (s) + H2SO4 (l) à 2 HCl (g) + Na2SO4 (s) Method 2: Reaction of hydrogen and chlorine gas
H2 (g) + Cl2 (g) à 2 HCl (g)
Most common method: Byproduct of other synthetic processes
A common acid in industrial and domestic settings (hardware store: Muriatic Acid)
Removal of rust from steel surfaces “pickling”
Purification of glucose and corn syrup
Manufacture of Cl–containing chemicals
Preparation of Ionic Halides (Metal Halides)
Method 1: Metal + halogen (produces metal in high oxidation state, e.g. Fe3+)
2 Fe + 3 Cl2 → 2 FeCl3
ox. # 0 0 +3 –1
Cl is the oxidizing agent (it is reduced to Cl–)
The high oxidation state of Fe3+ results from the strong oxidizing ability of Cl2. (recall Cl2 = sufficiently strong oxidant to abstract 3 electrons from Fe)
Method 2: Metal + HCl (produces metal in low oxidation state; e.g. Fe2+)
Fe + 2 HCl → FeCl2 + H2
ox. # 0 +1 –1 +2 –1 0
H+ is the oxidizing agent (it is reduced to H2)
The low oxidation state of Fe2+ results from the weak oxidizing ability of H+ which can only abstract 2 e- (1 for each H+)
Preparation of Hydrated Ionic Halides
Metal oxides + HCl in water give hydrated metal halides
This is just acid–base chemistry again: acid + base ® salt + water
MgO (s) + 2 HCl (aq) + 5 H2O (l) → MgCl2•6H2O
base acid salt water
Most metal halides are water-soluble.
Exception: many metal fluorides are insoluble
e.g. CaCl2 water soluble
CaF2 insoluble
Why? F– is small and has high charge density • small, high charge density cation + anion: the salt has a high lattice energy. • dissolution becomes thermodynamically unfavorable
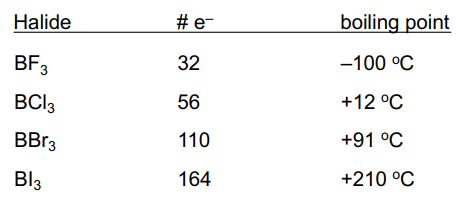
Covalent Halides
have low boiling points
intermolecular forces are weak, as compared to ions and metals
Boiling point trend above is related to strength of dispersion forces.
Greater # electrons → stronger dispersion forces
Preparation of Covalent Halides
Reaction of nonmetal with dihalide.
Often several oxidation states of the product are possible. The product depends on stoichiometry: how many equivalents of non-metal used?
e.g. 2 P (s) + 5 Cl2 (g) → 2 PCl5 (s)
2 P (s) + 3 Cl2 (g) → 2 PCl3 (l)
If the nonmetal is inert (e.g. N2), an alternative synthesis is necessary:
N2 (g) + 3 Cl2 (g) → 2NCl3 (l)
NH3 (g) + 3 Cl2 (g) → NCl3 (l) + 3 HCl (g)
Reactivity of Covalent Halides
Most covalent halides react vigorously with water
PCl3 (l) + 3 H2O (l) → H3PO3 (l) + 3 HCl (g)
BUT, covalent fluorides are often kinetically inert
e.g. CF4, SF6 do not react with water Neither does CCl4… too small; OH2 can’t get at the carbon
Metal halides can have covalent bonding, if high charge density, larger anion
SnCl4 (l) + 4 H2O (l) → Sn(OH)4 (s) + 4 HCl (g)
Reactivity with water is a sign of covalent bonding Absence of reactivity could mean it's ionic... or just barrier too high (eg CHCl3) Lesson: be cautious about negative evidence
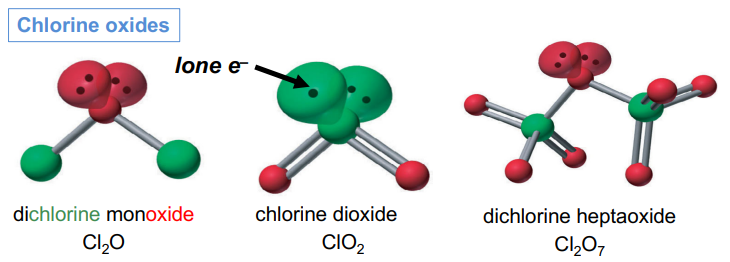
Halogen Oxides, XnOm Chlorine
Chlorine forms many oxides: wide range of oxidation states
Cl2O , ClO2, ClO3, Cl2O7
+1 +4 +6 +7
Cl is often central
Smaller size of O (2nd vs 3rd period), lone-pair compression add instability if central O
ClO2 is the only one produced on large scale: for wood bleach, water purification
Generated on-site by treating the chlorite NaOCl with strong acid
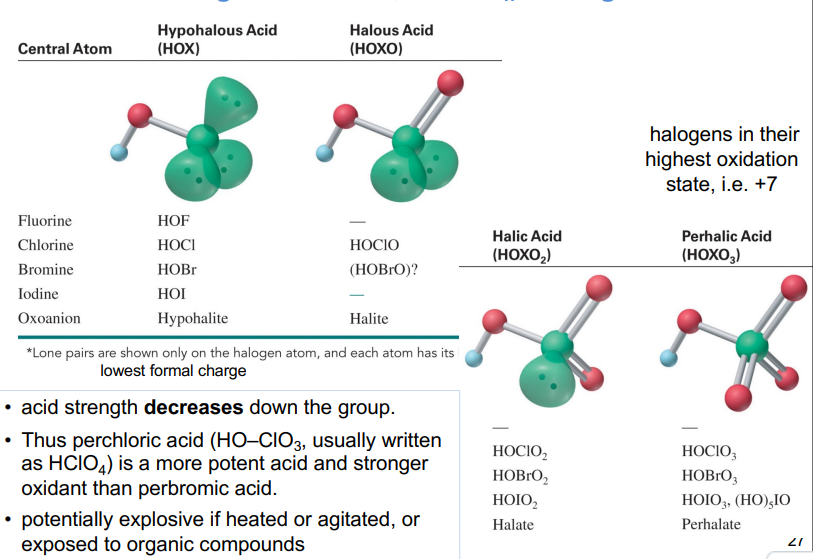
Halogen Oxoacids, HO–XOn: strong oxidants
Relative Strength of Halogen Oxoacids
relative strength of halogen oxoacids depends on both the electronegativity and the oxidation state of the halogen
For oxoacids with different halogens in the same oxidation state, acid strength decreases as the halogen EN decreases
ie order of acidity is HOClO2 > HOBrO2 > HOIO2
For oxoacids of a given halogen, acid strength decreases as the halogen oxidation state decreases.
ie order of acidity is HOClO3 > HOClO2 > HOClO
Oxoacid anions
The relative strength depends on both the electronegativity and the oxidation state of the halogen
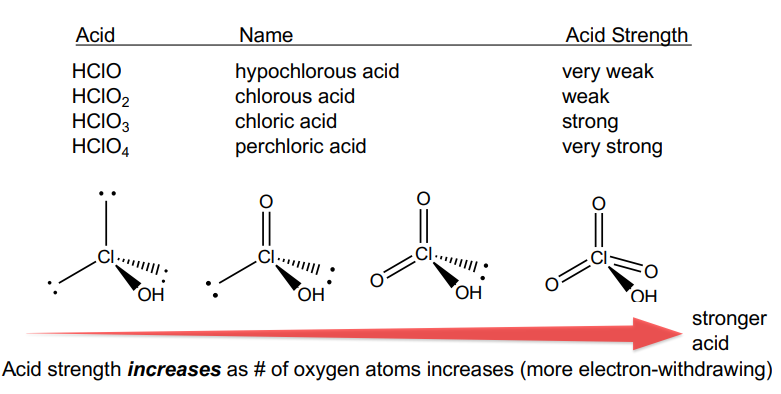
Oxyacids of Chlorine
In all cases, the acidic hydrogen is actually bound to oxygen – the conventional molecular formula is MISLEADING
Hypochlorous Acid (HClO, HOCl) and Hypochlorite Ion (ClO–)
HClO (better written HOCl) is a weak acid. Thus, ClO– is basic.
HOCl is formed on dissolving Cl2 in water (as discussed earlier)
Cl2 + H2O → HCl (g) + HOCl (aq) Hypochlorous acid
HOCl (aq) + H2O (l) → H3O+ (aq) + ClO- Hypochlorite
strong oxidizing capabilities of HOCl makes it useful as bleach and bactericide (etc.)
Recall NaOCl, sodium hypochlorite = LIQUID bleach Ca(OCl)2, calcium hypochlorite = bleach powder
NaOCl + 2 HCl → HOCl + NaCl
HOCl + HCl → Cl2 + H2O
Perchloric Acid (HClO4) Forms Perchlorate Ion (ClO4 –)
The strongest acid of all simple acids. Pure perchloric acid can explode unpredictably
Very Strong Oxidizer:
• Contact with organic materials (wood, paper) causes immediate fire!
• Can oxidize metal alloys to generate the metal ions
KClO4 is used in fireworks and flares
NH4ClO4 (ammonium perchlorate) is used in solid rocket boosters, with Al:
6 NH4ClO4 (s) + 8 Al (s) → 4 Al2O3 (s) + 3 N2 (g) + 3 Cl2 (g) + 12 H2O (g)
One shuttle launch uses 850 tons of NH4ClO4 Phase change (solid à gas), plus exothermic reaction
Interhalogen compounds, XYn
For halogens bonded to each other (beyond diatomics): central atom has the lower electronegativity: it is in a +ve oxidation state
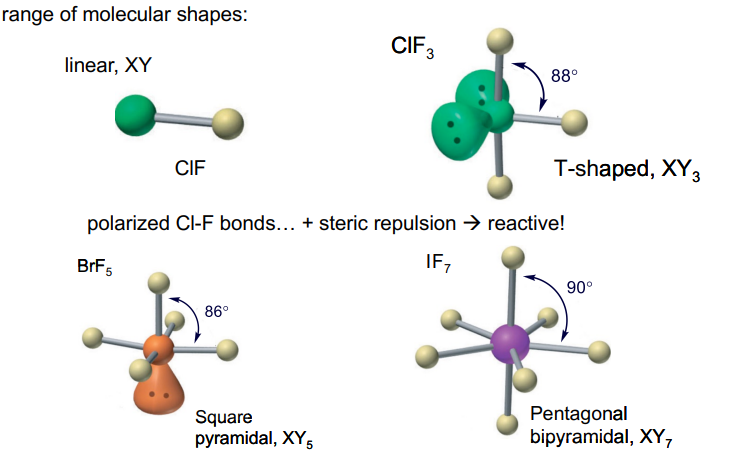
Interhalogen Compounds
odd–numbered groups have odd oxidation states; even–numbered groups have even oxidation states.
When bonds form or break, two electrons are typically involved, so the oxidation states of the atoms involved commonly change by 2
Odd–numbered oxidation states: F and I are both in (odd #) Group 17 0 +1 +1 +3
exception to I2 + F2 → 2 IF typical IF + F2 → IF3
OS = 2 0 -1 OS change -1 0 -1
Even–numbered oxidation states: S is in Group 16 (even #)
0 +2 +2 +4
S + F2 → SF2 SF2 + F2 → SF4
Hydrolysis of XYn gives a covalent halide HY + “XO” acid (X does not change oxidation state) e.g. ClF3 → HF + HClO2