GI FINAL MASTER SET
1/200
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
201 Terms
What are the major functions of cholesterol and its derivatives?
Modulates membrane fluidity; essential for function of receptors, pumps, channels.
Precursor for:
Bile acids (lipid emulsification, digestion, absorption)
Steroid hormones
Vitamin D
Intermediates serve as precursors for:
Coenzyme Q (ubiquinone)
Molecules involved in development (defects → CHILD, SLOS syndromes)
High cholesterol in β‑cells → decreased insulin secretion.
Many pathogens rely on cholesterol for cell entry/replication.
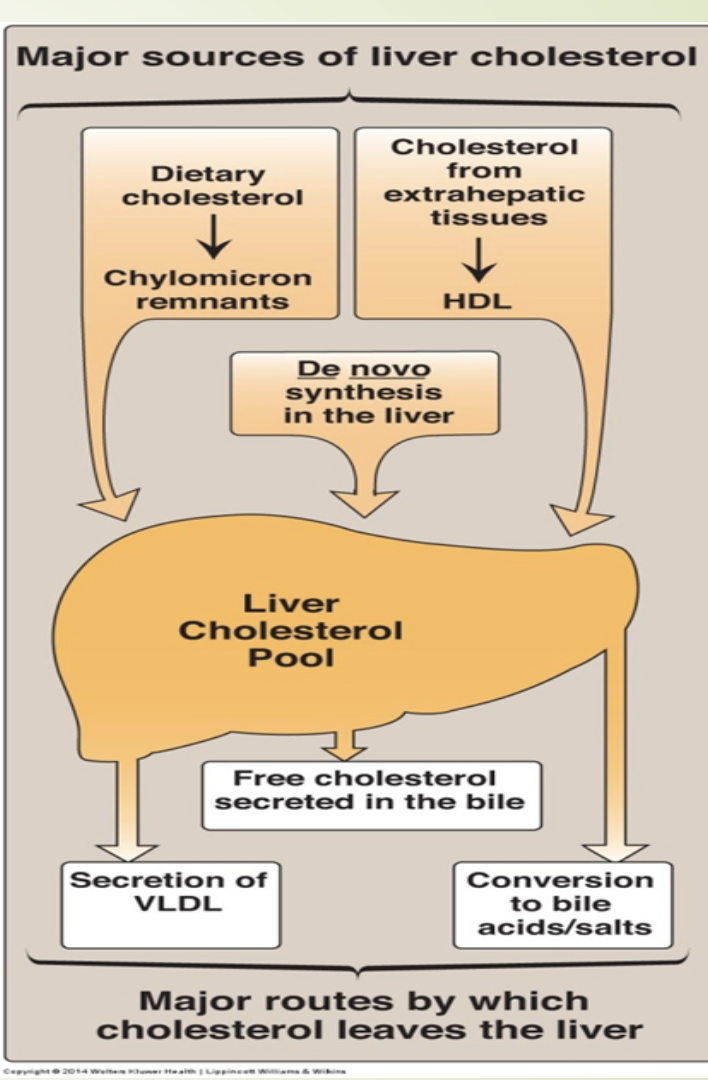
What are the dietary and endogenous sources of cholesterol?
Diet: ~400 mg/day; ~50% absorbed.
De novo synthesis: ~1.2 g/day.
All tissues can synthesize cholesterol.
Major sites: liver, intestine, adrenal cortex, reproductive tissues.
What are the structural features of cholesterol?
Steroid nucleus (A–D rings).
Nearly fully saturated (one double bond).
Planar ring structure.
One hydroxyl group (site of esterification).
Hydrophobic molecule with small polar head.
What are the requirements and cellular location for cholesterol synthesis?
Requires large amounts of energy.
NADPH provides reducing power.
ATP provides additional energy.
Occurs in cytosol; some enzymes bound to ER membrane.
Starting molecule: Acetyl‑CoA from:
β‑oxidation of fatty acids
Pyruvate dehydrogenase
Ketogenic amino acids
What are the major intermediates in cholesterol synthesis?
Acetyl‑CoA (C2)
Mevalonate (C6)
Isoprenoid units (C5)
Squalene (C30)
Lanosterol (C30)
Cholesterol (C27)
Rate‑limiting enzyme: HMG‑CoA reductase.
What is the committed step in cholesterol synthesis and how is it regulated?
Begins with condensation of 3 acetyl‑CoA → HMG‑CoA.
Committed step: HMG‑CoA → mevalonate.
Catalyzed by HMG‑CoA reductase (ER‑bound).
Major control point for cholesterol biosynthesis.
How is HMG‑CoA reductase regulated short‑term?
Inhibited by phosphorylation
Low ATP → AMP‑activated protein kinase → phosphorylation → inhibition
Glucagon promotes phosphorylation → inhibition
Activated by dephosphorylation
Insulin activates protein phosphatase → dephosphorylation → activation
High cholesterol & bile acids bind HMG‑CoA reductase → increase susceptibility to proteolytic degradation → decreased cholesterol synthesis.
How does SREBP regulate HMG‑CoA reductase expression?
SREBP binds sterol regulatory element (SRE) → increases transcription.
Exists in complex with SCAP.
High cholesterol:
SCAP‑SREBP stays in ER
No Golgi translocation
No cleavage → no active transcription factor
↓ HMG‑CoA reductase expression
Low cholesterol:
SCAP‑SREBP moves to Golgi
Cleaved by S1P and S2P proteases
Active SREBP enters nucleus → ↑ transcription of HMG‑CoA reductase and other lipid genes.
How are cholesterol esters synthesized and why is this important?
Catalyzed by ACAT (Acyl‑CoA cholesterol acyltransferase).
Transfers fatty acid from fatty‑acyl‑CoA → cholesterol ester.
Occurs in most cells.
Prevents toxic accumulation of free cholesterol in membranes.
Most absorbed cholesterol is esterified before entering chylomicrons.
Plays a role in atherosclerosis (foam cell formation).
What is the mechanism of statins and their effects on cholesterol levels?
Competitive inhibitors of HMG‑CoA reductase.
Examples: lovastatin, simvastatin, atorvastatin, rosuvastatin.
Effects:
↑ LDL receptor synthesis
↑ LDL uptake by liver
↓ LDL concentration by 20–60%
↓ cardiovascular disease risk
Grapefruit juice increases circulating statin levels
What are the major side effects of statins and why are they taken at night?
Side effects:
↓ Coenzyme Q synthesis → myopathy, muscle weakness
Rhabdomyolysis → kidney injury
Circadian rhythm:
Cholesterol synthesis peaks ~6 hours after dark
HMG‑CoA reductase expression follows same rhythm
Statins taken at night maximize inhibition.
How is cholesterol synthesis linked to Coenzyme Q?
Coenzyme Q (ubiquinone) is synthesized from intermediates of cholesterol synthesis.
Statin inhibition of HMG‑CoA reductase → ↓ CoQ → muscle toxicity.
What are the major functions of bile acids?
Reduce cholesterol content in circulation (cholesterol → bile acids).
Emulsify lipids → aid digestion & absorption.
Prevent cholesterol precipitation in gallbladder.
Stimulate intestinal motility.
Enhance pancreatic lipase activity.
Aid absorption of fat‑soluble vitamins (A, D, E, K).
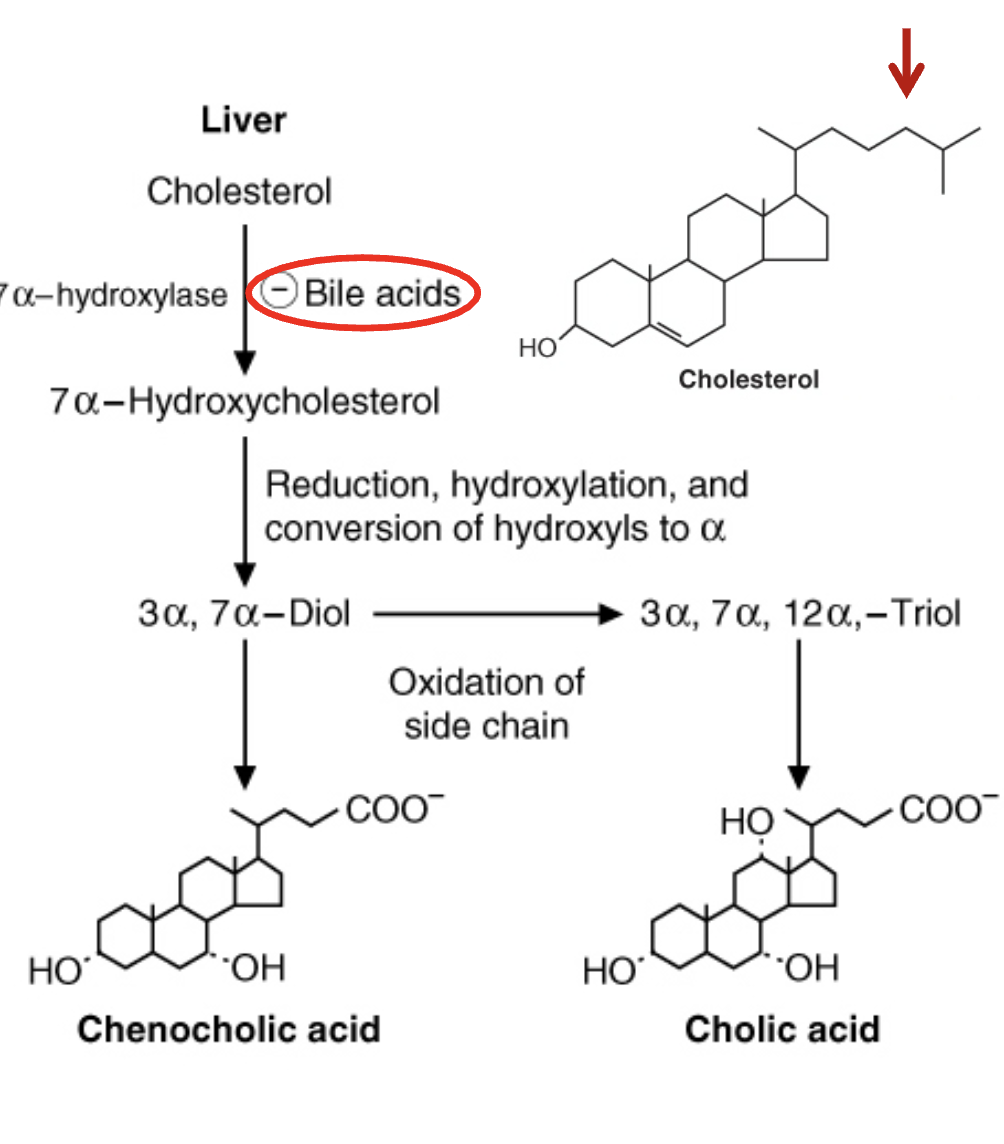
How are bile acids synthesized and regulated?
Synthesized in liver.
More water‑soluble than cholesterol.
Rate‑limiting enzyme: 7α‑hydroxylase.
Inhibited by bile acids (end‑product inhibition).
Expression regulated by circadian rhythm.
pKa ~6 → 50% protonated at intestinal pH.
“Bile acids” and “bile salts” often used interchangeably; salts = ionized forms.
What are conjugated bile acids and why are they important?
Formed by conjugation of cholic acid with glycine or taurine.
Better detergents than unconjugated forms.
Lower pKa (~4) → almost fully ionized at intestinal pH.
More effective in lipid digestion and absorption.
How are secondary bile acids formed and what are their properties?
Intestinal bacteria deconjugate and dehydroxylate bile salts.
Removal of 7‑OH group → secondary bile acids.
Less soluble; lower reabsorption rate.
Reabsorbed secondary bile acids are reconverted to primary bile acids in liver.
How do bile acid sequestrants lower cholesterol?
Bind bile acids/salts in the intestine → prevent reabsorption.
Liver must synthesize more bile acids → uses cholesterol.
↓ Liver cholesterol → ↑ LDL receptor expression.
↑ LDL uptake by hepatocytes → ↓ blood cholesterol
Bile contains bile acids, cholesterol, lipids, bilirubin.
Reduce fat abs ie drug or diet, water retention
How is cholesterol excreted from the body?
Sterol ring cannot be degraded by humans.
Excreted as:
Biliary cholesterol
Bile acids
Most bile acids are reabsorbed via enterohepatic circulation.
This reduces the need for high‑rate synthesis and allows large secretion into intestine.
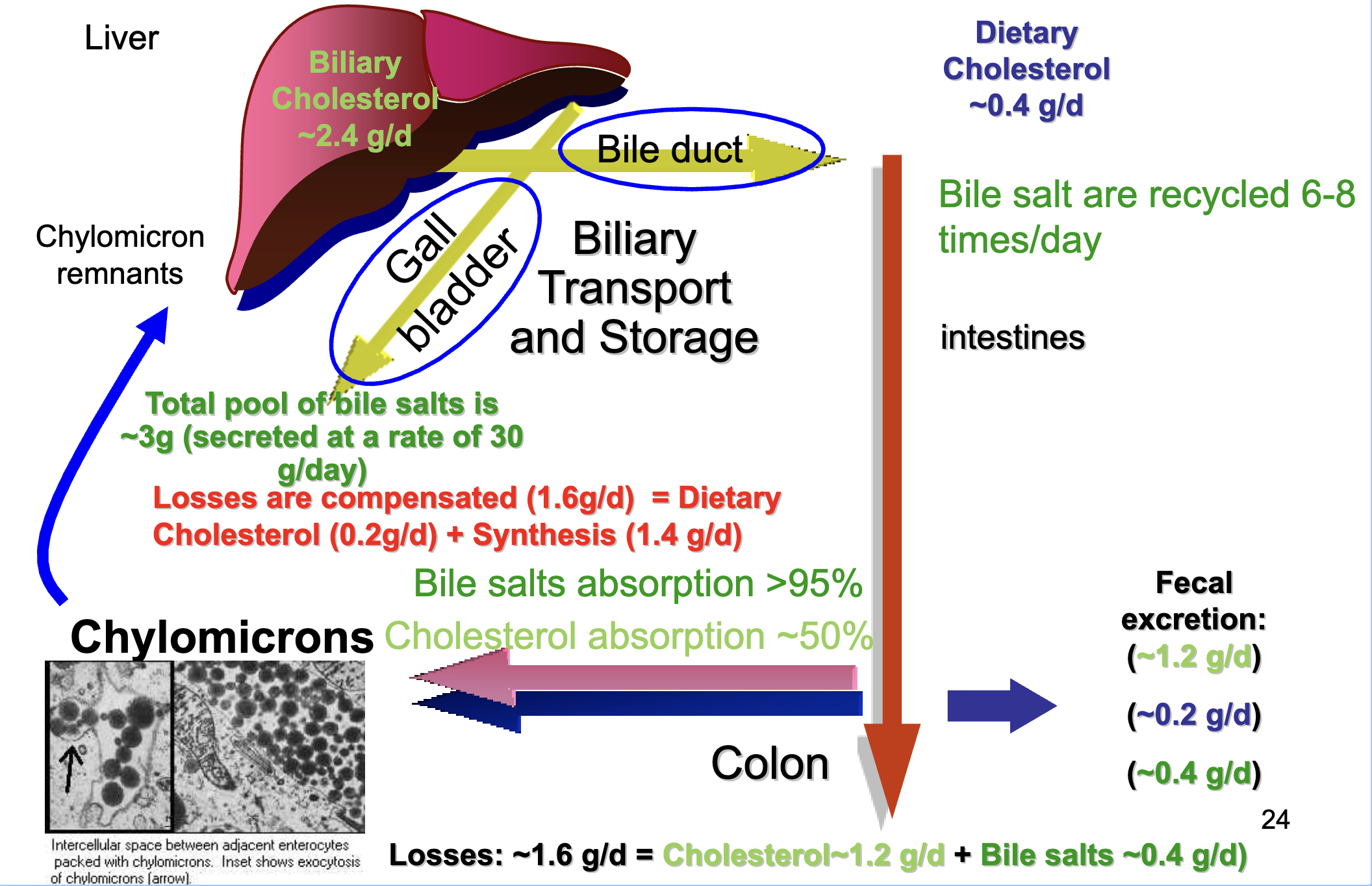
What are the key features of enterohepatic circulation? Understand idea
Total bile salt pool ≈ 3 g, secreted 30 g/day.
Bile salts recycled 6–8 times/day.
Bile salt absorption >95%.
Cholesterol absorption ~50%.
Daily losses ≈ 1.6 g/day (cholesterol 1.2 g + bile salts 0.4 g).
Losses replaced by:
Dietary cholesterol (0.2 g/day)
De novo synthesis (1.4 g/day)
What factors contribute to cholesterol gallstone formation?
Occur in up to 20% of Western populations.
80% are cholesterol stones.
Cholesterol solubility maintained by phospholipids + bile salts.
Risk increases when:
Cholesterol : bile salts/phospholipids ratio > 1:1 → cholesterol crystallizes.
Decreased gallbladder contraction → prolonged retention → crystallization.
What causes bile salt malabsorption and what are its consequences?
Causes: Crohn’s disease, celiac disease, chronic pancreatitis.
Consequences:
High bile acids in colon → water secretion → chronic diarrhea.
Poor absorption of fat‑soluble vitamins (A, D, E, K).
Cholestasis:
Bile cannot flow from liver to duodenum.
Obstructive: gallstones, infection, malignancy.
Metabolic: genetic defects in bile formation.
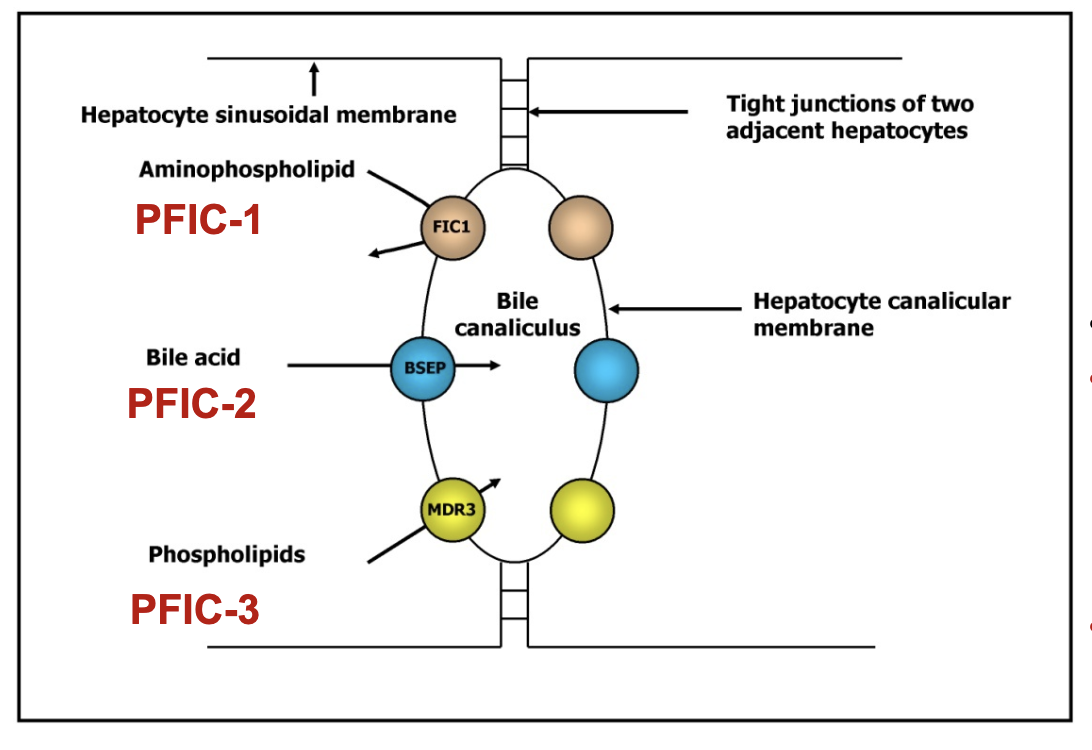
What are the types of PFIC and their molecular defects?
PFIC‑1 (Byler disease):
Mutation: ATP8B1 (FIC‑1) for ATPase FIC-1
Defective phospholipid translocation
Symptoms: short stature, deafness, diarrhea, pancreatitis, low fat‑soluble vitamins, early liver failure
PFIC‑2:
Mutation: ABCB11 (BSEP)
Defective bile salt export pump → bile salt retention → cholestasis
PFIC‑3:
Mutation: ABCB4 (MDR3) for multidrug resistant protein 3 (MDR3) or floppase
Lack of phosphatidylcholine in bile → free bile acids damage biliary epithelium → liver disease
All PFIC: cholestasis, jaundice, failure to thrive, intense pruritus.
What are the key summary points of cholesterol metabolism?
Cholesterol is a membrane component and steroid precursor.
Synthesized from acetyl‑CoA; liver & intestine are major producers.
HMG‑CoA reductase is rate‑limiting; regulated short‑ and long‑term.
Intermediates have important biological functions.
Primary bile acids synthesized in liver; 7α‑hydroxylase is rate‑limiting.
Bile acids aid digestion, absorption, and cholesterol balance.
Enterohepatic circulation maintains cholesterol homeostasis.
Gallstones form when cholesterol exceeds solubilizing capacity.
Cholestasis may be obstructive or metabolic.
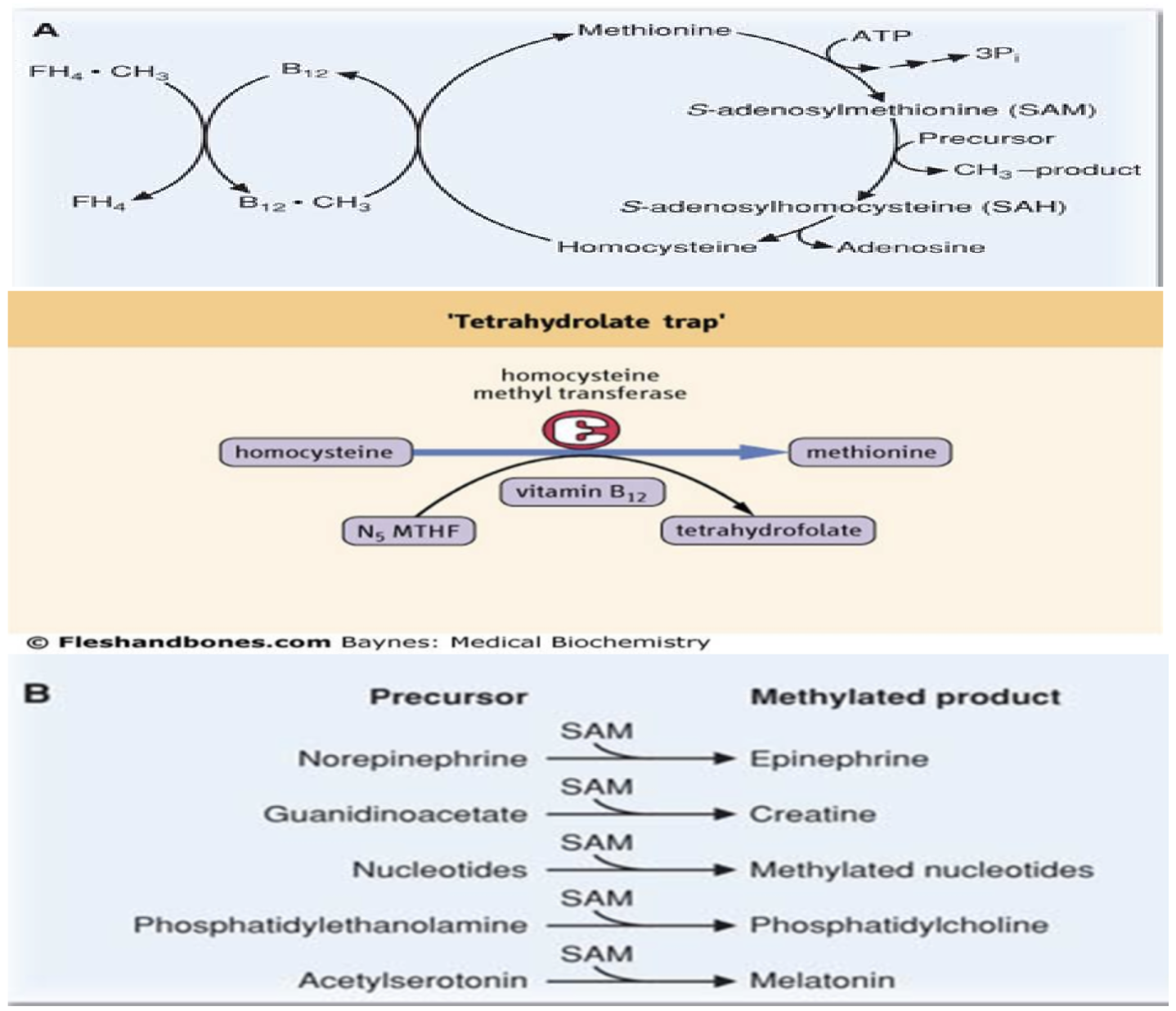
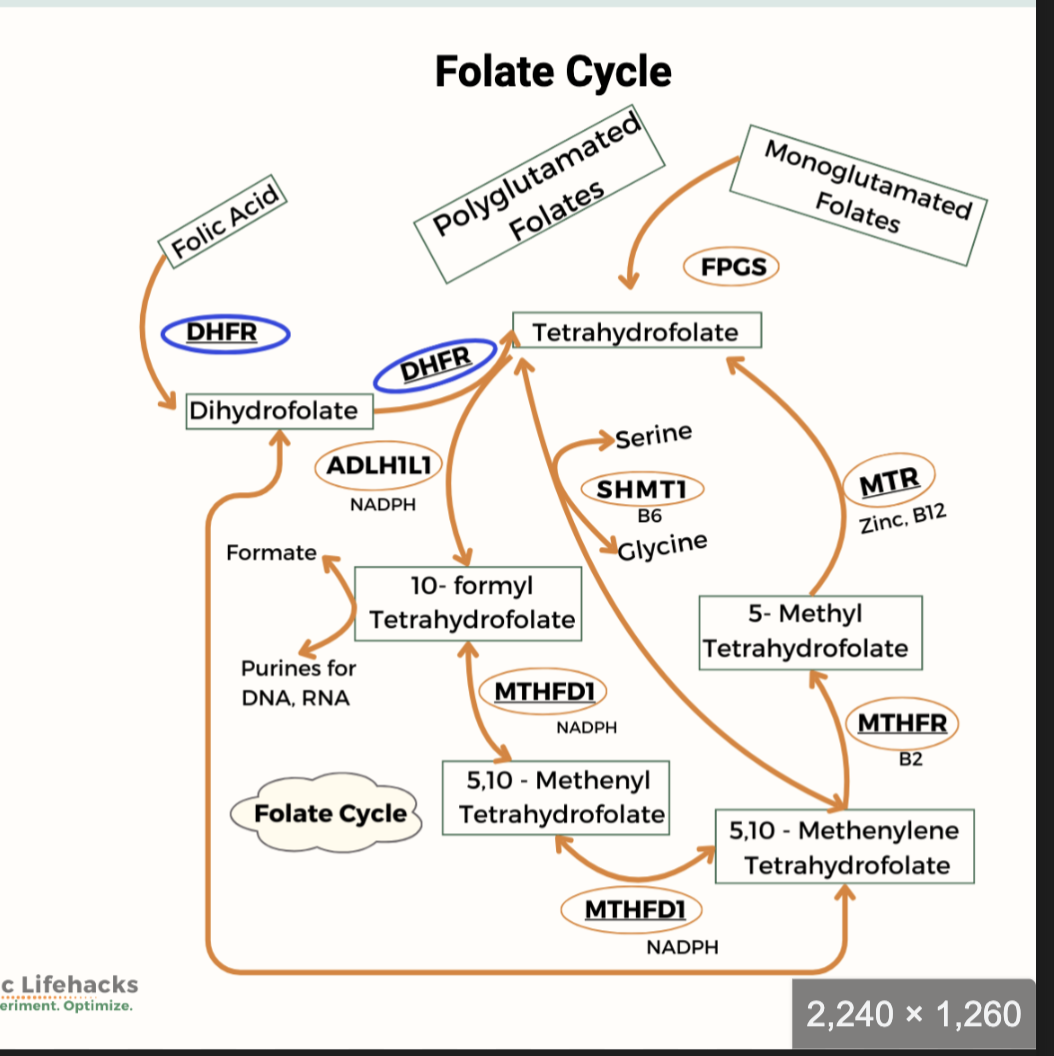
What relationship exists between folate, vitamin B12, and SAM?
What is the function of folate in bone marrow and what is its coenzyme form?
Bone marrow produces billions of RBCs and leukocytes per hour requiring:
Nucleotides
Amino acids
Iron
Folic acid is essential for one‑carbon transfer reactions.
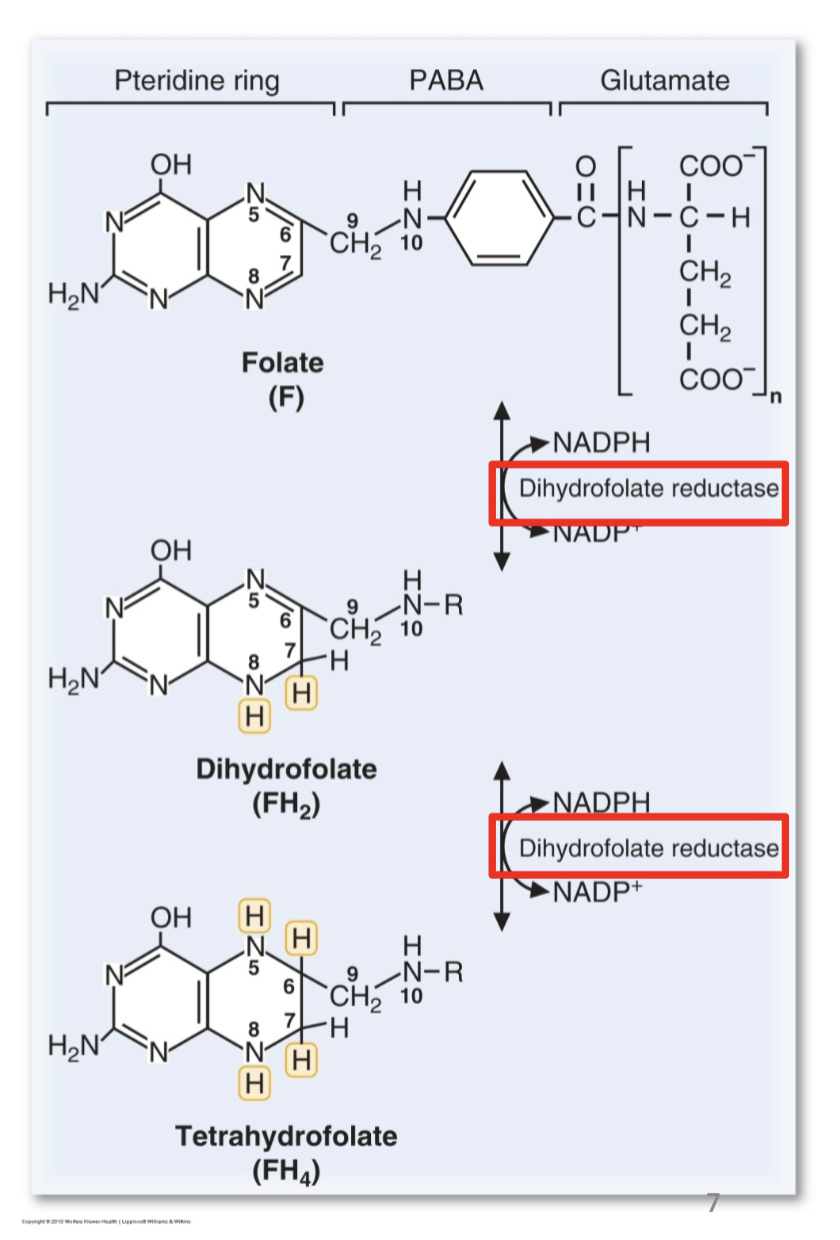
Coenzyme form: tetrahydrofolate (FH4).
What reactions involve FH4 and what are the active forms of folate?
FH4 participates in one‑carbon transfer reactions:
1. Amino acid interconversion:
Gly → Ser
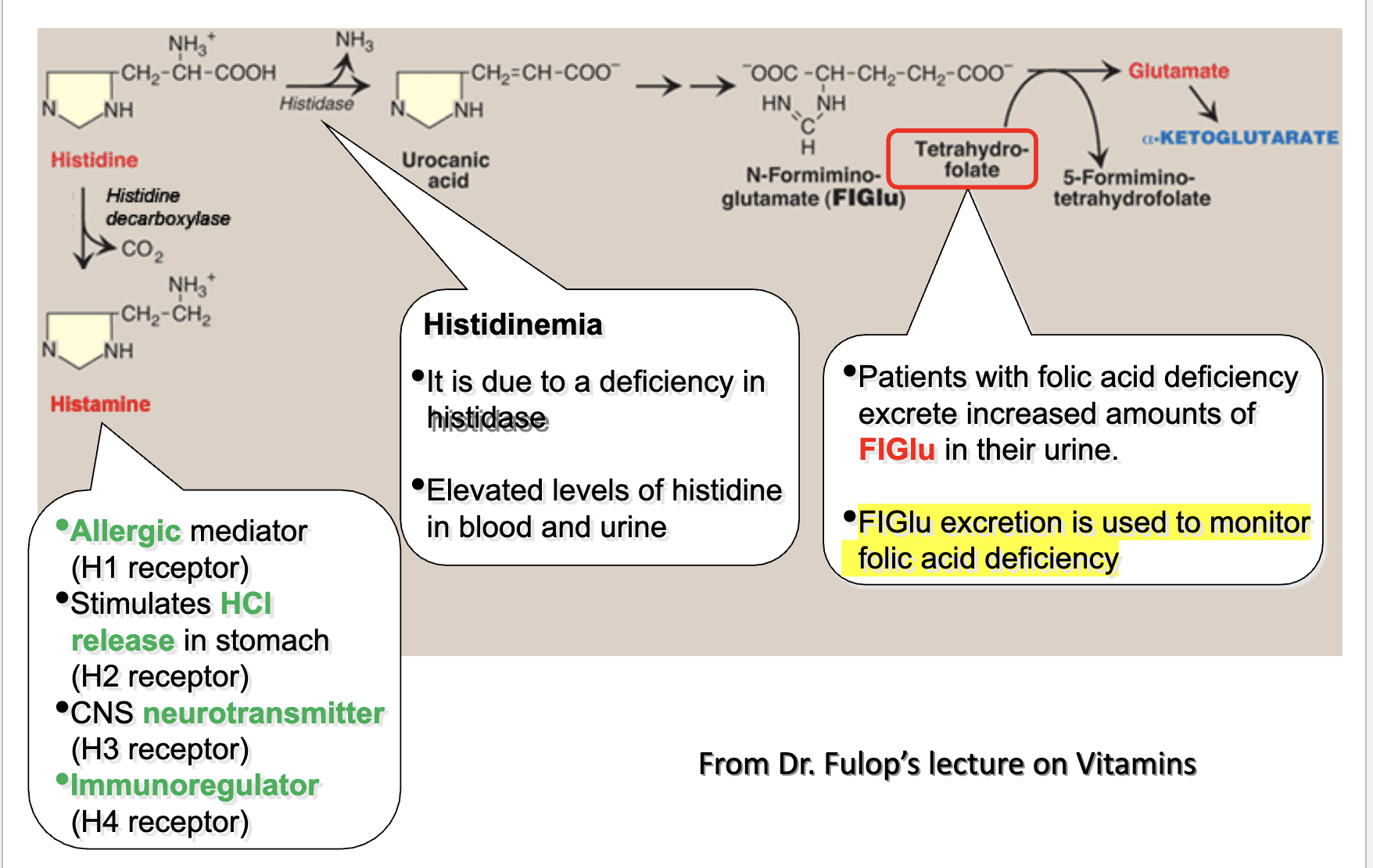
His → Glu (FIGLU accumulates when folate deficient; FIGLU measured in urine)
Homocysteine → Methionine (requires folate + B12; “trap reaction”)
2. DNA synthesis:
Purines
Pyrimidine base thymine
Active forms of folate:
N5‑methyl‑FH4
N10‑formyl‑FH4 (purine synthesis)
N5,N10‑methylene‑FH4 (pyrimidine synthesis)
Additional notes:
N5,N10‑methylene‑FH4 reductase polymorphism common in US
FIGLU is an intracellular biomarker and helps distinguish folate vs B12 deficiency
What are the key points about folate reduction to FH4?
DHFR catalyzes both reduction steps.
Multiple glutamate residues are added intracellularly.
Plants synthesize folate; humans cannot → dietary requirement.
PABA is a precursor.
“R” represents the portion of the folate molecule to the right of N10.
Why is folate targeted in cancer therapy and what is methotrexate?
Folate is required for DNA synthesis.
Methotrexate is a folate analog.
It is a competitive inhibitor of DHFR (key enzyme in pyrimidine biosynthesis).
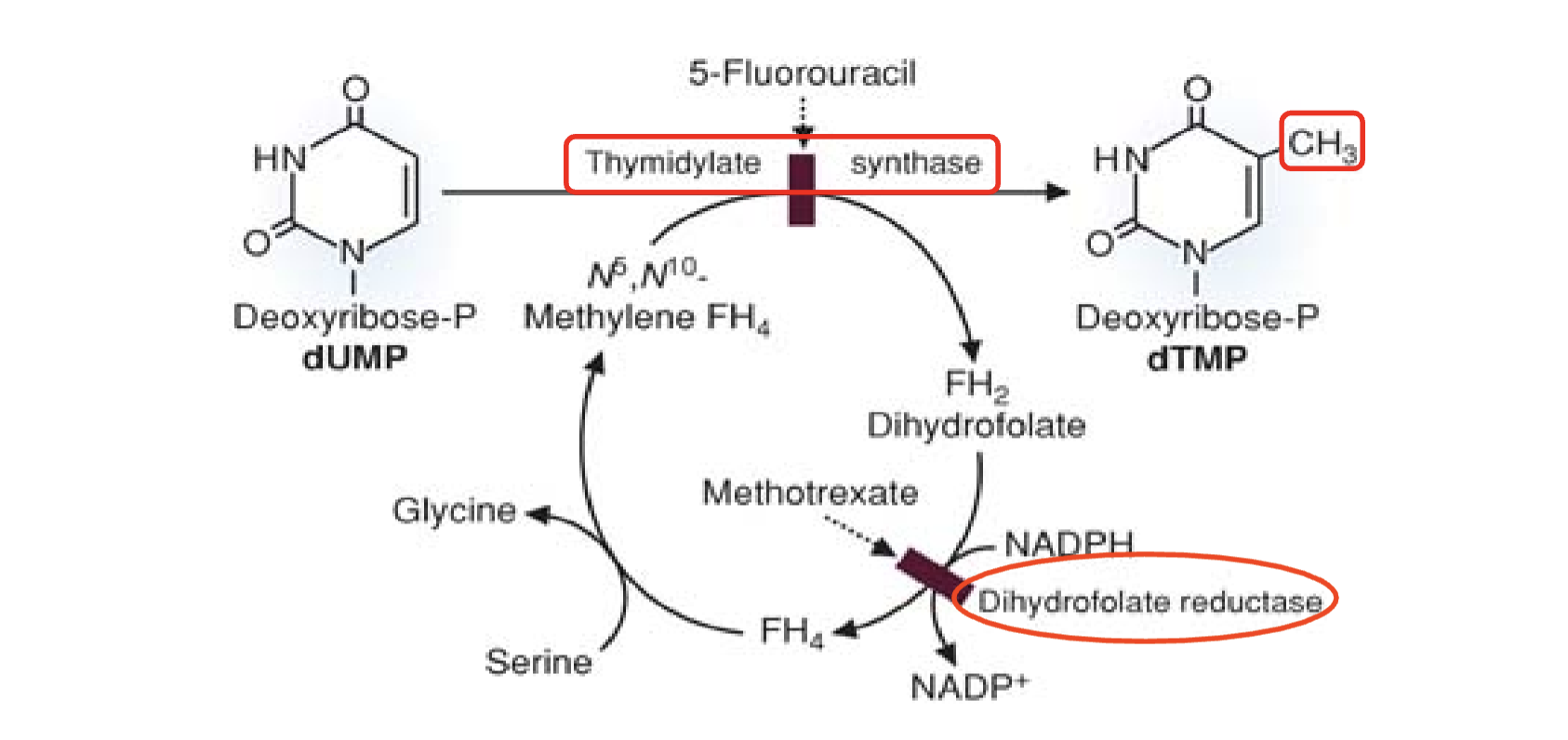
What does Figure 40.5 illustrate about folate metabolism and drug targets?
N5,N10‑methylene‑FH4 donates a one‑carbon unit to dUMP → dTMP.
FH4 oxidized to FH2.
FH2 reduced back to FH4 by DHFR.
FH4 regenerated to N5,N10‑methylene‑FH4 using serine.
Drug inhibition sites:
5‑FU inhibits thymidylate synthase
Methotrexate inhibits DHFR
Inhibiting dUMP → dTMP starves cancer cells of thymidine, halting DNA synthesis and triggering cell death.
What diseases are treated with methotrexate and what are its side effects?
Uses:
Rheumatoid arthritis/Psoriasis/Asthma/Alcoholic cirrhosis/Inflammatory bowel disease
Side effects:
GI distress (diarrhea)
Severe folate deficiency → anemia
Folate supplementation reduces toxicity
What are the nutritional sources of folate and how does bioavailability vary?
Biological availability varies with the source.
Best sources:
Liver
Fortified cereals
Legumes (garbanzo beans/chickpeas, lentils)
Vegetables (leafy greens)
Less rich sources:
Eggs
Oranges
What are the causes of folate deficiency and how is it detected?
Causes:
Low dietary intake
Alcoholism (inadequate absorption)
Folic acid antagonists (methotrexate, trimethoprim)
Oral contraceptives
Giardia infection
Celiac disease
Pregnancy/Psoriasis (increased requirement)
Old age
Compromised utilization (Vitamin B12 deficiency)
Excessive excretion (long-standing diarrhea)
Detection:
Serum folate
RBC folate (reflects long-term stores)
Alcohol intake may transiently decrease serum folate despite adequate stores
High urinary FIGLU indicates folate deficiency
How is histidine metabolism used to assess folate deficiency?
Histidinemia results from histidase deficiency → elevated histidine in blood/urine.
In folate deficiency, FIGLU excretion increases.
FIGLU excretion is used to monitor folic acid deficiency.
Basis: His → FIGLU → Glu requires folate.
What are the hematologic and fetal consequences of folate deficiency?
Early RBC synthesis in bone marrow is first affected.
Disrupts WBC synthesis.
Neural tube defects in fetus:
Spina bifida
Anencephaly
Supplemental folic acid late in pregnancy may correlate with childhood asthma (studies cited).
How does folate deficiency lead to megaloblastic anemia?
RBCs cannot replicate DNA.
Cells grow large but cannot divide → megaloblasts.
Megaloblasts retain nuclei.
Fewer mature RBCs released → decreased oxygen-carrying capacity → megaloblastic anemia.
What are the clinical signs and symptoms of folate deficiency?
Inflammation of tongue and mouth
Glossitis
Stomatitis
Abnormal skin pigmentation
Weakness
Tiredness
Diarrhea
Poor growth
Depression
Mental confusion
What are the two major reactions requiring vitamin B12?
Regeneration of tetrahydrofolate:
Homocysteine + N5‑methyl‑FH4 → methionine + FH4
Catalyzed by methionine synthase/homocysteine methyltransferase
Lack of methionine → progressive demyelination → neurological disorders
Conversion of methylmalonyl‑CoA → succinyl‑CoA:
Catalyzed by methylmalonyl‑CoA mutase
Basis of the Schelling test
Diagnostic note:
B12 deficiency → ↑ MMA + ↑ homocysteine
Folate deficiency → ↑ homocysteine only
What is pernicious anemia and what causes it?
Pernicious anemia = vitamin B12 deficiency causing death within 2–5 years if untreated.
Juvenile form: defective intrinsic factor (IF) production by gastric parietal cells.
Symptoms resemble folate deficiency anemia (megaloblasts).
True B12 deficiency results from impaired absorption due to lack of IF.
What are the causes of true vitamin B12 deficiency?
Absence of intrinsic factor
Defective pancreatic protease synthesis
Bacterial overgrowth in small intestine
Tapeworm infestation
Antiulcer medications (reduced acid synthesis)
Chronic malabsorption syndromes (AIDS)
Chronic pancreatic disease
Surgical removal of ileum or stomach
Crohn’s disease (Crohn ileitis)
What are the most nutrient-dense dietary sources of vitamin B12?
Organ meats (liver, kidneys, heart)
Seafood
Beef
Eggs
Hot dogs (contain organ meat scraps)
Ham
Milk and milk products
What additional clinical signs and symptoms occur in pernicious anemia due to vitamin B12 deficiency?
Nerve degeneration
Sensory disturbances in legs (tingling, numbness)
Paresthesia (burning, prickling)
Neurological disease when chronic
Loss of concentration
Memory loss
Visual disturbances
Disorientation
Dementia
Sore tongue
Severe cases: loss of bowel and bladder control
What reactions require SAM and how is SAM synthesis linked to folate and B12?
SAM is an essential methyl donor for synthesis of:
Norepinephrine → Epinephrine
Guanidinoacetate → Creatine
Nucleotides → Methylated nucleotides
Phosphatidylethanolamine → Phosphatidylcholine
Acetylserotonin → Melatonin
Methionine is the methyl donor for SAM synthesis.
Methionine is produced from homocysteine.
Vitamin B12 is required for homocysteine → methionine conversion.
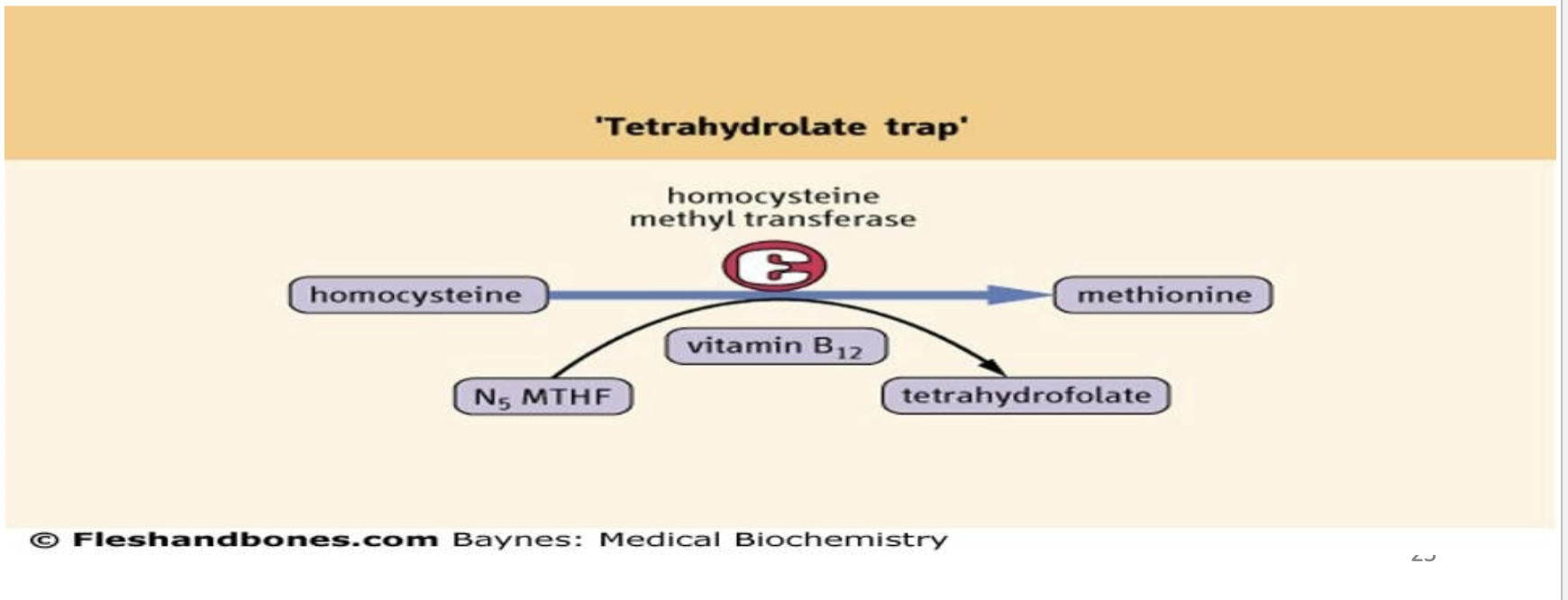
What is the methyl trap hypothesis and what are its consequences?
Without vitamin B12, THF becomes trapped as N5‑methyl‑FH4 (MTHF).
This causes shortage of usable folate coenzyme forms → inhibition of DNA synthesis.
Vitamin B12 deficiency leads to secondary folate deficiency.
Homocysteine accumulates → homocystinuria.
How does hyperhomocysteinemia arise in folate or B12 deficiency?
Homocysteine is derived from S‑adenosylhomocysteine after SAM donates a methyl group.
Deficiency of folate or B12 prevents remethylation of homocysteine to methione → accumulation.
What hematologic condition results from deficiency of either folate or vitamin B12?
Megaloblastic anemia.
Measurement of blood folate and vitamin B12 determines the cause.
What are the metabolic fates of homocysteine and which vitamins are required?
Homocysteine can be:
Methylated to methionine (requires folate + vitamin B12)
Condensed with serine to form cystathionine (requires vitamin B6/pyridoxal phosphate)
Cystathionine → cysteine.
Cysteine accumulation inhibits cystathionine synthesis → homocysteine accumulation → release into bloodstream.
Deficiency of folate, B12, or B6 → high homocysteine → neurological disease (demyelination) + cardiovascular disease (blood clots).
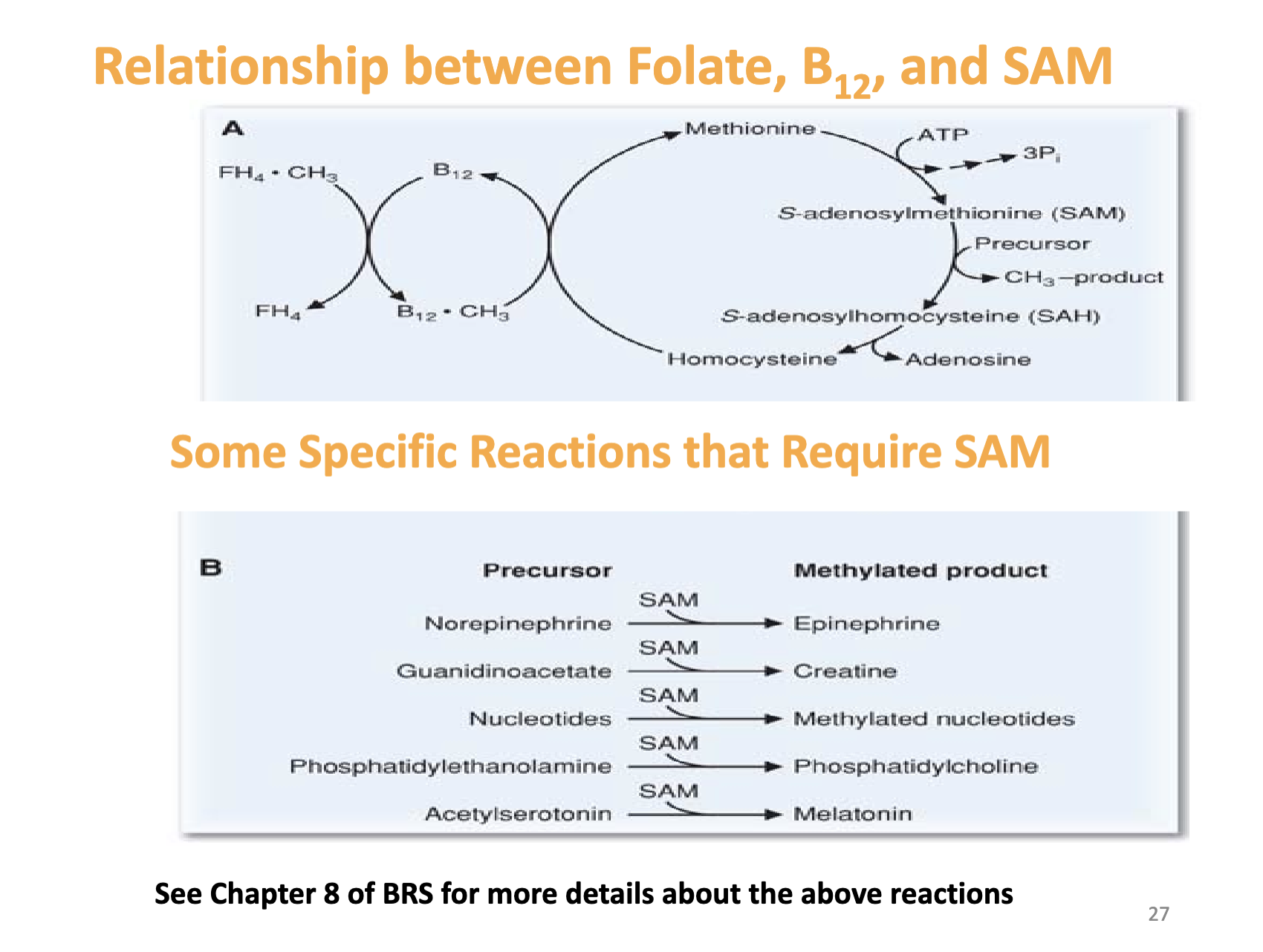
What does this slide emphasize about SAM‑dependent reactions?
Lists specific reactions requiring SAM.
Notes to see Chapter 8 of BRS for more details.
Reinforces the interconnected roles of folate, B12, and SAM in methylation pathways.
How is folate deficiency linked to neural tube defects and what genetic mutation affects folate metabolism?
Folate coenzyme deficiency during pregnancy increases risk of neural tube defects.
Folate supplementation before conception and for at least 1 month after reduces risk.
Mutation in N5,N10‑methylene‑FH4 reductase → elevated homocysteine due to decreased N5‑methyl‑FH4.
Mutation makes enzyme thermolabile → inhibits DNA synthesis.
What does the 1st law of thermodynamics state in relation to the body’s energy?
The body’s energy inputs must balance the sum of the energy outputs. Energy can neither be created nor destroyed.
How is basal metabolic rate (BMR) defined and what factors influence it?
What are the determinants of metabolic energy expenditure?
BMR = minimum rate of energy production to sustain vital functions in waking state.
Factors: age, gender, weight, hormonal status.
Resting metabolism (60–75%): BMR, sleeping metabolism, arousal metabolism
Physical activity (15–30%): at work, at home, sports and recreation
Feeding thermogenesis (10%)
Other factors: thyroid hormone, growth hormone, androgens, climate, aging, sleep, fever, stress, starvation
What are the two components of feeding energy expenditure?
Obligatory thermogenesis: energy requiring processes related to assimilating food (motility, secretion, digestion, absorption)
Facultative thermogenesis: related to activation of endocrine (insulin/glucagon) and autonomic nervous systems and their stimulating effect on metabolic substrate mobilization (glycogenolysis and lipolysis), storage (glycogenesis and lipogenesis) or processing (gluconeogenesis).
ermic effect of food is maximal 1 hr postprandial.
What are the hunger and satiety centers and how do they function?
Hunger center: located in lateral hypothalamic area; stimulation elicits voracious appetite even after ingestion of adequate food.
Satiety center: located in ventromedial nucleus (VMN); stimulation elicits sensations of satiety even in the presence of food.
od intake is regulated by hypothalamic centers.
What conclusions were drawn from parabiosis experiments involving Ob and Db mice?
What roles do leptin and leptin receptors play in Ob and Db mice?
Db mouse makes an excess of a blood borne factor that cures Ob.
Db mouse lacks receptor for this factor.
Absence of the receptor in the Db mouse removes negative feedback leading to high levels of the blood borne factor.
Wildtype mouse has leptin, and this helps the Ob mouse feel “satisfied” and not overeat.
The Db mouse lacks the leptin receptor, therefore overeats and overproduces leptin. Leptin enters the Wt mouse and its effect on satiety causes the Wt mouse to starve.
Since the Db mouse makes plenty of leptin, its overproduction helps cure the Ob mouse.
What circulating hormones and signals affect the satiety and hunger centers?
Leptin: Adipocytes are primary producers of leptin. Leptin levels rise in proportion to the mass of adipose tissue. Acute changes in food intake or fasting do not appear to appreciably affect leptin levels. Leptin provides long-term feedback on body status.
Insulin: Levels change dramatically daily in response to food intake. Insulin provides short-term feedback on body status.
What are the anorexigenic effects of leptin?
Stimulation of leptin receptors decreases fat storage.
Decreased production in the hypothalamus of appetite stimulators, such as NPY and AGRP.
Activation of POMC neurons, causing release of α-MSH and activation of melanocortin receptors.
Increased sympathetic nerve activity, which increases metabolic rate and energy expenditure.
Decreased insulin secretion by pancreatic beta cells, which decreases energy storage.
Adipose tissue uses leptin as a signal to the brain that enough energy has been stored and that intake of food is no longer necessary.
Obesity may be associated with leptin resistance due to defective leptin receptors or post receptor signaling pathways normally activated by leptin.
What are the two types of arcuate nucleus neurons that control energy balance?
POMC neurons release α-MSH and CART, decreasing food intake and increasing energy expenditure.
AGRP and NPY neurons increase food intake and reduce energy expenditure.
AGRP acts as an antagonist of MCR-4.
Insulin, leptin, and CCK inhibit AGRP-NPY neurons and stimulate POMC-CART neurons.
Ghrelin activates AGRP-NPY neurons and stimulates food intake.
What are the key features of ghrelin as an orexigenic signal?
Ghrelin is a hormone released mainly by cells of the stomach.
Blood levels of ghrelin rise during fasting, peak just before eating, and then fall rapidly after a meal.
Ghrelin stimulates secretion of growth hormone for GH metabolic effects.
How do CCK and stretch signals contribute to satiety?
CCK activates receptors on local sensory nerves in the duodenum, sending messages to the NTS via the vagus nerve that contribute to satiation and meal cessation.
The effect of CCK is short-lived and chronic administration has no major effect on body weight.
CCK prevents overeating during meals but may not play a major role in meal frequency or total energy consumed.
How does glucose act as an anorexigenic signal?
A rise in blood glucose increases firing of gluco-receptor neurons in the satiety center (ventromedial and paraventricular nuclei).
The same increase decreases firing of gluco-sensitive neurons in the hunger center of the lateral hypothalamus.
Some amino acids and lipid substances also affect firing of these neurons.
What mechanisms maintain normoglycemia after a meal?
Suppression of hepatic glucose production
Stimulation of hepatic glucose uptake
Stimulation of glucose uptake by peripheral tissues (muscle)
How do major organs handle glucose after a meal?
Liver stores glucose as glycogen and converts some to FAs, packaged as VLDLs for export to adipocytes.
Muscle stores glucose as glycogen and converts some to lactate and gluconeogenic amino acids for export to the liver.
Adipocytes convert glucose to glycerol-3-phosphate, a precursor of TAGs.
How do major organs handle amino acids after a meal?
Liver converts gluconeogenic amino acids to glycogen.
Muscle converts amino acids to protein.
How are fats captured and stored after a meal?
Chylomicrons undergo hydrolysis in systemic blood vessels.
Lipoprotein lipase on vascular endothelial cells makes FA available to adipocytes.
Adipocytes re-esterify FAs with glycerol-3-phosphate (from glucose) for storage as TAGs.
What are the major steps of energy liberation (catabolism)?
Breakdown of glycogen or TAGs to simpler compounds
For carb catabolism, step 2 is glycolysis
For TAG, step 2 is β-oxidation
The final common step for oxidizing carb, TAG and proteins to CO₂ are TCA cycle and oxidative phosphorylation.
How do epinephrine and glucagon mobilize energy stores?
A) In muscle, epinephrine promotes glycogenolysis and glycolysis, producing ATP for contraction and lactate.
B) In liver, primarily glucagon and also epinephrine trigger glucose production in the short term via glycogenolysis, and over the long term via gluconeogenesis. Hepatocytes can generate glucose and export it to the blood because they have G6Pase.
C) In adipocytes, epinephrine triggers production of FAs and glycerol, which leave the adipocytes and enter the blood.
What are the priorities and features of fasting metabolism?
Priority #1 is stable supply of energy for CNS function in the form of glucose or ketone bodies. Blood-brain barrier impermeable to FAs.
In fed state and early fasting, glucose is oxidized to meet CNS demands.
Other major organs oxidize FAs.
During prolonged fasting (>2 days), liver metabolizes FAs to raise levels of ketones for CNS use.
Priority #2 – maintain protein reserves.
What metabolic processes occur during an overnight fast?
After a fast, decline in insulin shifts metabolism to FA mobilization.
Body still metabolizes glucose at 7–10 g/hr.
Free glucose only about 15–20 g (2 hours).
Body must produce glucose at a rate to match ongoing consumption.
4–5 hr postprandial fall in insulin and increase in glucagon signal liver to initiate glycogenolysis and gluconeogenesis, each contributing ~50%.
Gluconeogenesis: Cori cycle (lactate/pyruvate), glucose-alanine cycle (alanine, glutamine).
Lipolysis: fall in insulin permits release of FA and glycerol from adipocytes.
What metabolic changes occur beyond an overnight fast?
Low insulin shifts liver to gluconeogenesis.
Muscle accelerates proteolysis to contribute glycogenic AA (alanine and glutamine).
Low insulin activates HSL so more FA and glycerol released from adipocytes.
Increase in FA causes insulin resistance in muscle interfering with GLUT4.
Prolonged fast: hepatic gluconeogenesis falls and shifts to renal gluconeogenesis (up to 40%).
Body decreases use of protein for gluconeogenesis.
Hypoinsulinemia and high glucagon increase hepatic oxidation of FAs that increase liver ketogenesis.
How are ketones synthesized and used during prolonged fasting?
Synthesized in the liver from FAs.
Liver does not have β-ketoacyl CoA transferase, so acetoacetate and D-β-hydroxybutyrate enter the bloodstream.
CNS, skeletal muscle, and cardiac muscle consume acetoacetate or D-β-hydroxybutyrate to produce 2 acetyl-CoA molecules that enter the TCA cycle.
What metabolic changes occur during prolonged starvation?
As fat stores are depleted, levels of leptin decrease.
Low leptin affects the hypothalamic-pituitary-gonadal axis decreasing LH and FSH causing anovulation.
Protects fertile women in times of famine.
What are the three phases of protein depletion during starvation?
Initial rapid depletion from use of easily mobilized protein for direct metabolism or conversion to glucose for brain metabolism.
Slow depletion: gluconeogenesis decreases to 1/3–1/5 previous rate → excessive fat utilization → ketone body production → brain uses ketones → partial preservation of protein stores.
With fat stores almost depleted, protein becomes the only remaining energy source → rapid depletion → death when proteins are depleted to about half their normal level.
What happens to fat and carbohydrate stores during starvation?
Progressive depletion of tissue fat and protein.
Fat is the prime source of energy (100× more fat energy stored than CHO), so fat depletion continues until most fat stores are gone.
Tissues preferentially use carbohydrate for energy but stores are small, providing only enough energy for half a day.
What is a KUB and what does it evaluate?
What does KUB show in urinary tract evaluation?
KUB = Kidneys, Ureters, Bladder.
AP, recumbent; may add upright view.
Used for: gas patterns, air‑fluid levels, free air.
Must include hemidiaphragms → pubic symphysis.
Requires knowledge of normal organ locations.
Urolithiasis: calcium, oxalate, urate, cysteine, phosphate stones.
Stones <4 mm → 80% pass; 5 mm → 20% pass.
IVP: outlines kidneys/ureters/bladder; used for stones, prostate issues, tumors; largely replaced by CT.
Intravenous Pyelogram (IVP)
What are key points about foreign body ingestion?
Most FBs low‑risk; often no imaging needed.
Large or oropharyngeal objects → surgical review.
High‑risk: button batteries, magnets (corrode tissue).
Highest incidence: 6 months–3 years, developmental disorders.
Psychiatric patients may intentionally ingest FBs.
What is the role of contrast in abdominal plain films?
Barium outlines GI tract (oral or enema).
Detects: tumors, ulcers, hernias, diverticula, strictures, swallowing issues.
Small intestine: central, many loops, 3–5 cm, valvulae conniventes.
Large intestine: haustra, peripheral, >5 cm.
Barium enema → large intestine.
Useful for small bowel obstruction.
What are types and causes of bowel obstruction?
What are signs/symptoms of bowel obstruction?
Small bowel obstruction most common.
Mechanical: hernia, adhesions, volvulus, intussusception, tumor, mesenteric occlusion.
Non‑mechanical (ileus): anesthesia, nerve interruption, electrolyte imbalance, ischemia.
Abdominal pain (constant vs intermittent).
Distention (↑ with distal obstruction).
Hyper/hypoactive bowel sounds.
Nausea/vomiting.
Early diarrhea → late constipation.
Fullness, halitosis.
Fever, tachycardia.
Gross/occult blood.
History: surgery, neoplasm.
What does volvulus and dilated bowel look like on plain film?
Volvulus: twisted bowel + mesentery → obstruction.
Dilated loops: multiple dilated small bowel loops; some air in large bowel.
Large bowel obstruction → colon dilation.
What is intussusception and how does it appear?
Telescoping of bowel into itself → obstruction.
Medical emergency; most common in children.
May show soft tissue mass, obstruction signs.
What are key features of CT abdomen?
Axial slices (usually 5 mm).
High resolution; no structure overlap.
Contrast (oral/IV) improves visualization.
Includes pelvic structures.
Accuracy affected by metal, barium, stool, gas.
What does bowel obstruction look like on CT?
Dilated proximal bowel loops.
Transition point.
Collapsed distal bowel.
Possible cause: adhesions, hernia, volvulus, tumor.
Why is CT without contrast used for renal stones?
Stones are high‑density and easily visible without contrast.
CT can detect complications: hydronephrosis, ureteral dilation.
What are key features of AAA?
Potentially fatal if ruptured.
Usually between renal and iliac arteries.
Caused by atherosclerosis.
Often has calcific rim.
Often asymptomatic.
What are the advantages and uses of abdominal ultrasound?
No radiation; safe for pregnancy/pediatrics.
Real‑time imaging; Doppler for flow.
Used for: gallbladder, AAA, vessels, kidneys (hydronephrosis), liver, pancreas, spleen.
Stones may be seen; hydronephrosis seen better.
What is the FAST exam and what does it detect?
Focused Assessment with Sonography for Trauma.
Detects free fluid (internal bleeding).
Requires ~500 mL fluid to detect.
Not good for organ injury.
Extended FAST: pericardial effusion, pneumothorax, hemothorax.
How do CT and MRI compare?
CT: fast, good for acute issues, radiation, good for stones, trauma.
MRI: superior soft tissue detail, no radiation, slower, expensive, contraindications with metal.
What are the major functions of lipids and what diseases result from lipid imbalance?
Major source of energy (9 kcal/g)
Used in energy production as fatty acids
Hydrophobic barrier for subcellular compartmentalization
Regulators/cofactors (fat‑soluble vitamins)
Precursors of prostaglandins and steroid hormones
Stored in adipose tissue as triglycerides
Must be transported in lipoproteins
Disorders from imbalance: Obesity, Atherosclerosis → contribute to metabolic syndrome
What types of lipids are included in dietary lipid intake?
Triglycerides (esterified fatty acids)
Unesterified fatty acids (free fatty acids)
Cholesterol
Cholesteryl esters
Phospholipids
Where are chylomicrons synthesized and from what components?
Synthesized in intestinal mucosal cells
Made from dietary lipids and apoprotein B‑48
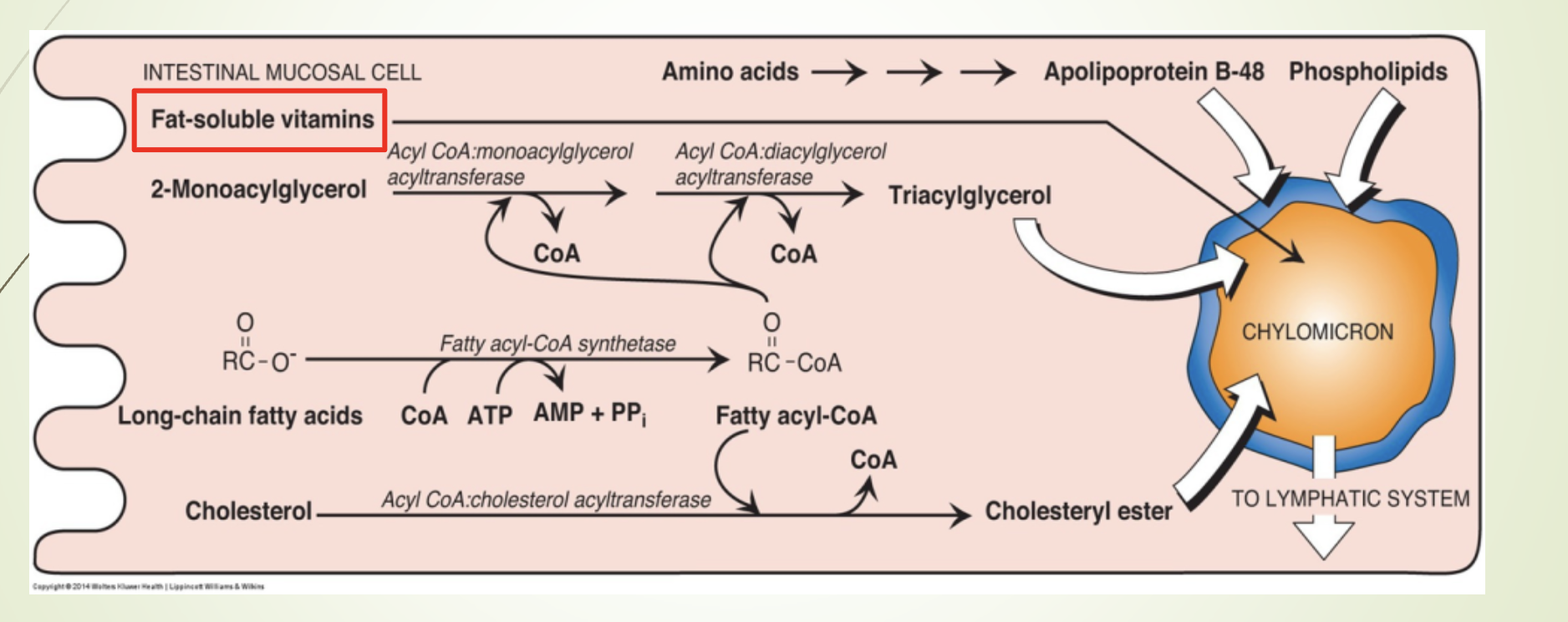
What is the function and composition of chylomicrons?
Function: Transport dietary lipids to peripheral tissues.
Composition:
82% triacylglycerols
Key apoprotein: Apo B‑48
MTP (MTTP) - Microsomal triglyceride transfer protein : Facilitates lipid binding to Apo B‑48
Maturation: Acquire Apo C (I, II, III) and Apo E from HDL (storage)
Release: Exocytosed into lymph → bloodstream
Note:
MTP deficiency → abetalipoproteinemia (no “B” type protein)
Autosomal recessive
Low/absent chylomicrons
Severe neuropathy, acanthocytosis, steatorrhea in infancy
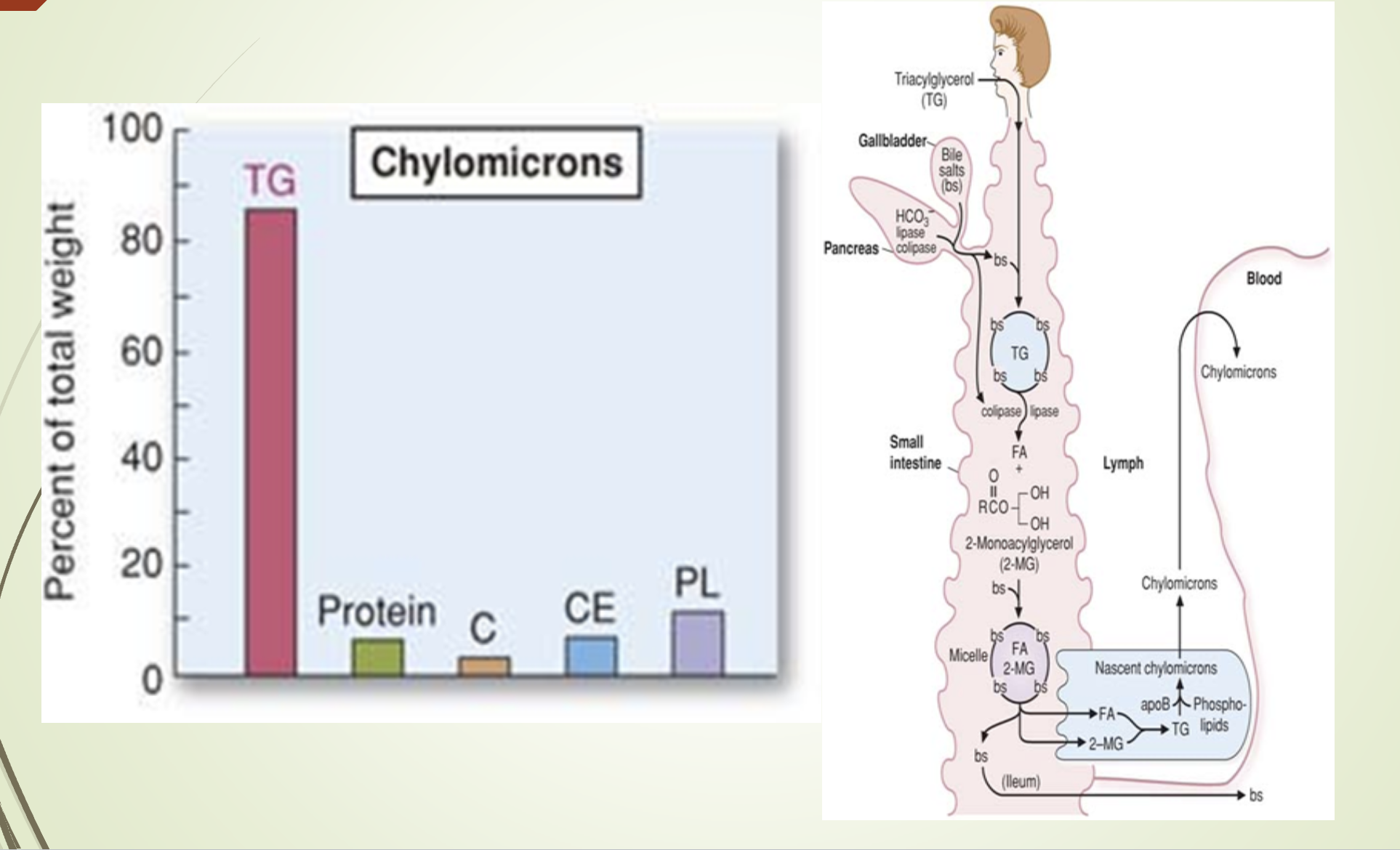
What does the chylomicron composition/synthesis slide emphasize?
Chylomicrons are synthesized in intestinal epithelial cells.
Composition dominated by triglycerides.
Structural apoprotein: Apo B‑48.
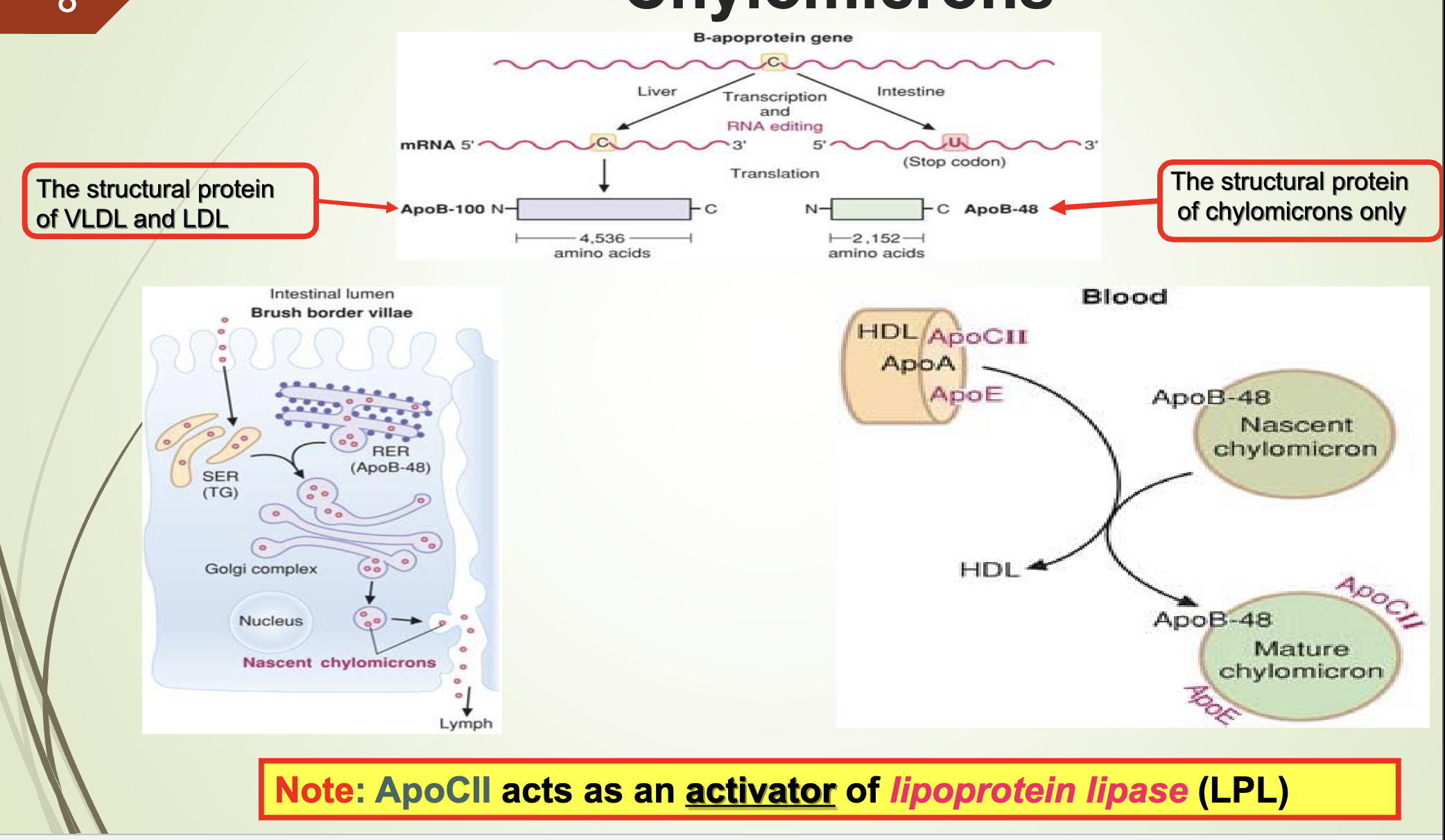
What apoprotein activates LPL and what are the structural proteins of VLDL/LDL vs chylomicrons?
Apo CII activates lipoprotein lipase (LPL).
Structural protein of VLDL & LDL: Apo B‑100
Structural protein of chylomicrons: Apo B‑48
What is the fate of triglycerides in chylomicrons and where is LPL located?
TGs are hydrolyzed by LPL → fatty acids + glycerol
LPL is located on luminal surface of capillaries in:
Muscle (especially cardiac)
Adipose tissue
Lactating mammary gland
Lungs
Kidneys
Liver - last, doesn’t process full chylomicrons
FFAs bind albumin for transport
FFAs: oxidized for ATP (muscle) or stored as TG ie esterfied (adipose)
What regulates LPL and what happens in diabetes or LPL deficiency?
Adipose LPL has highest Km; heart LPL lowest Km
LPL synthesis stimulated by insulin
Low insulin → accumulation of chylomicrons & VLDL
Insulin inhibits hormone‑sensitive lipase
Diabetes mellitus:
Low insulin → low LPL + high HSL → hypertriglyceridemia
Familial LPL deficiency (Type I hyperlipoproteinemia):
Autosomal recessive
Severe hypertriglyceridemia (>2000 mg/dL)
What happens to glycerol released from triglycerides during LPL action?
Glycerol is transported to the liver.
In the liver, glycerol is phosphorylated → glycerol‑3‑phosphate.
Glycerol‑3‑phosphate is used for:
Triglyceride synthesis in the fed state
Gluconeogenesis in the fasting state
Blood glycerol increases when TGs are hydrolyzed by LPL.
What is the fate of chylomicron remnants and what receptors are involved?
Remnants contain:
Cholesteryl esters
Phospholipids
Apolipoproteins
Small amount of TG
Remnants bind hepatocyte receptors via Apo E:
LDL receptor–related protein (LRP)
Remnants contain equal cholesterol and triglycerides.
Endocytosed and hydrolyzed in lysosomes.
How are chylomicron remnants processed after ApoE recognition?
ApoE binds LRP on hepatocytes.
Endocytosis → lysosomal degradation.
Products released into cytosol:
Fatty acids/Amino acids/Glycerol/Cholesterol
Type III hyperlipoproteinemia (dysbetalipoproteinemia):
Defective remnant removal
Autosomal recessive
Premature atherosclerosis
Xanthoma striata palmaris (palmar xanthomas)
Which lipidemia is more severe: ApoCII deficiency or ApoE deficiency?
ApoCII deficiency causes more severe lipidemia.
Because ApoCII is required to activate LPL, so TGs cannot be hydrolyzed at all. —> more severe
ApoE deficiency affects remnant clearance but does not block TG hydrolysis.
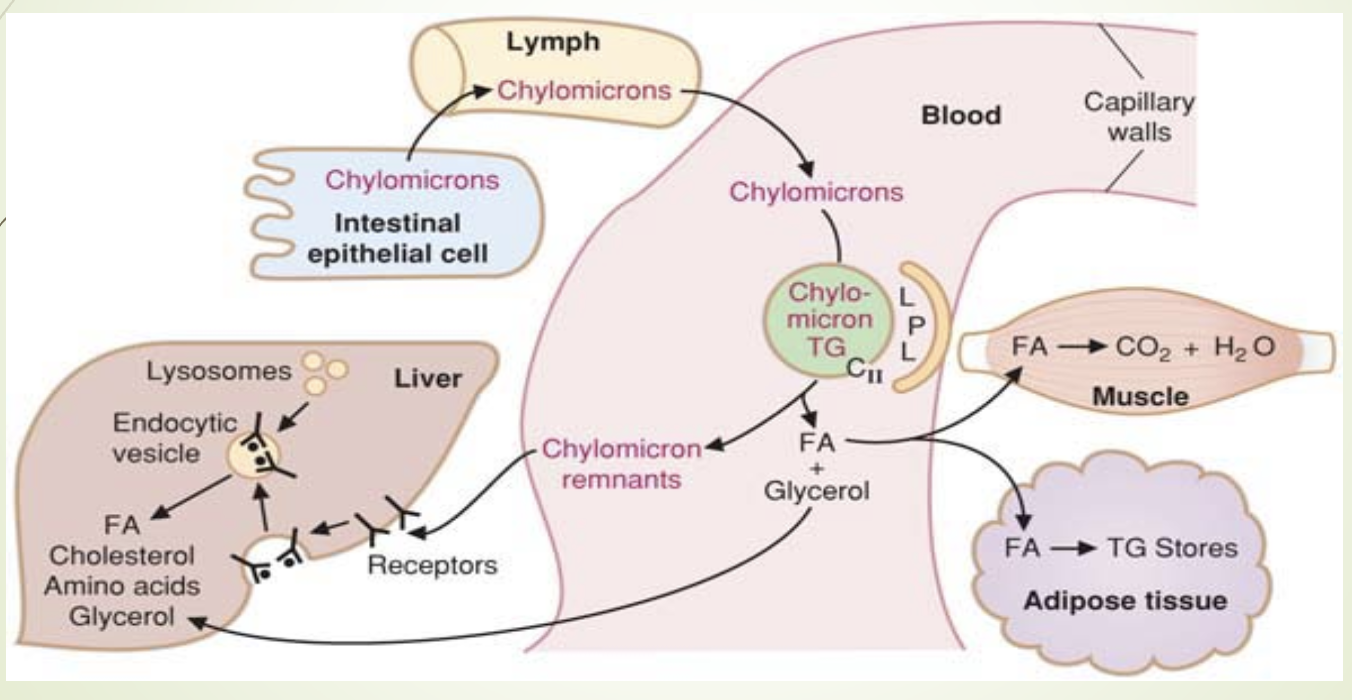
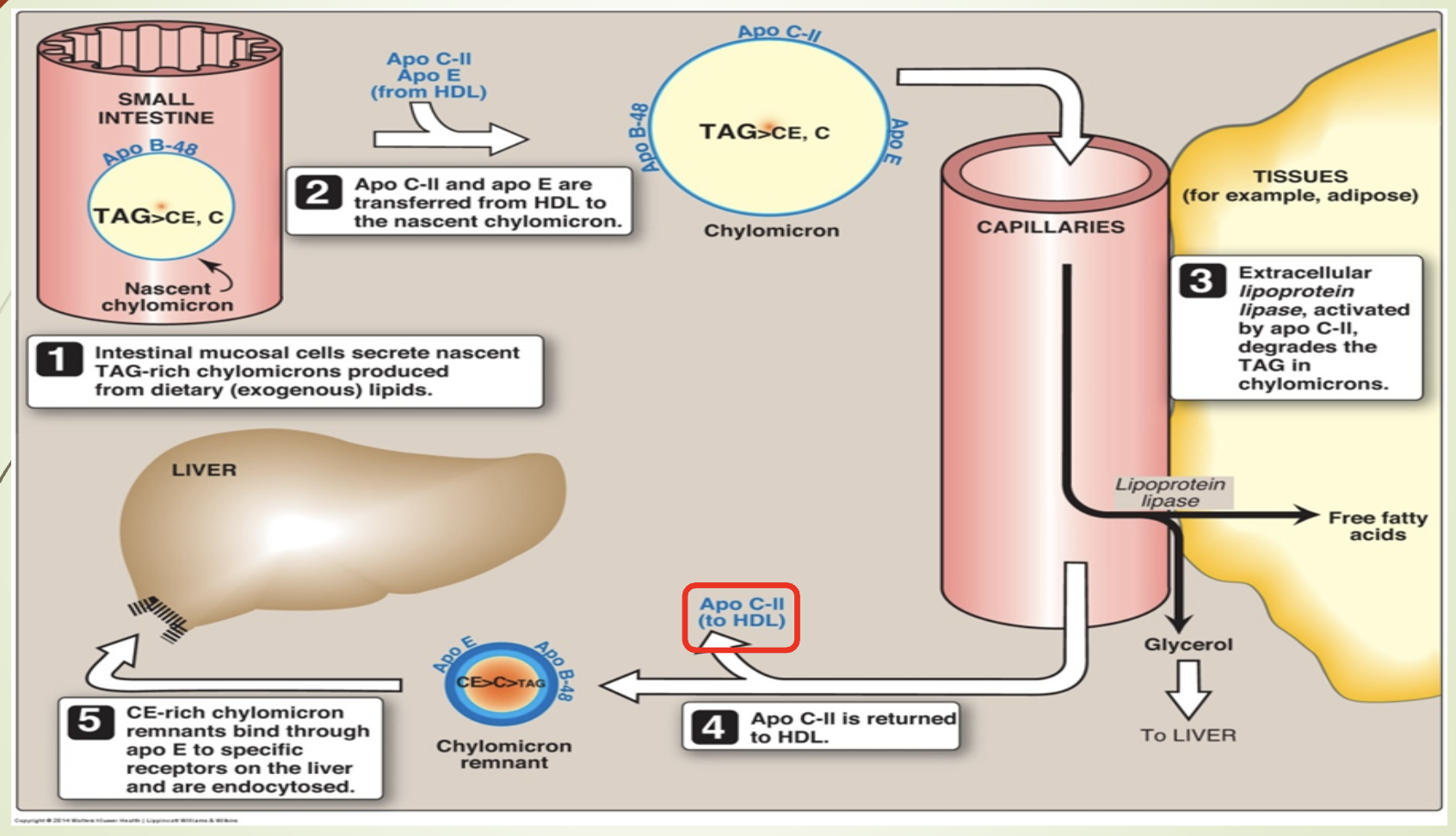
What does the chylomicron metabolism slide emphasize?
Synthesis in intestine → lymph → blood.
Maturation via ApoC and ApoE from HDL.
LPL (activated by ApoCII —> goes back to HDL) hydrolyzes TGs.
Remnants taken up by liver via ApoE‑LRP.
What is the composition, synthesis, and function of VLDL?
Composition:
52% TG (Hepatic aka endogenous)
22% cholesterol
18% phospholipids
8% apoproteins
Synthesis:
From chylomicron remnants
From TAGs not hydrolyzed in peripheral tissues
From dietary carbohydrates (major carbon source):
Glucose → acetyl‑CoA → fatty acids
Glucose → DHAP → glycerol
FA + glycerol → TAG
High‑carb intake → carbohydrate‑induced hypertriglyceridemia
Major apoprotein: Apo B‑100
Acquire ApoC and ApoE from HDL
MTTP loads TG onto ApoB100
Function: Transport hepatic lipids to peripheral tissues.
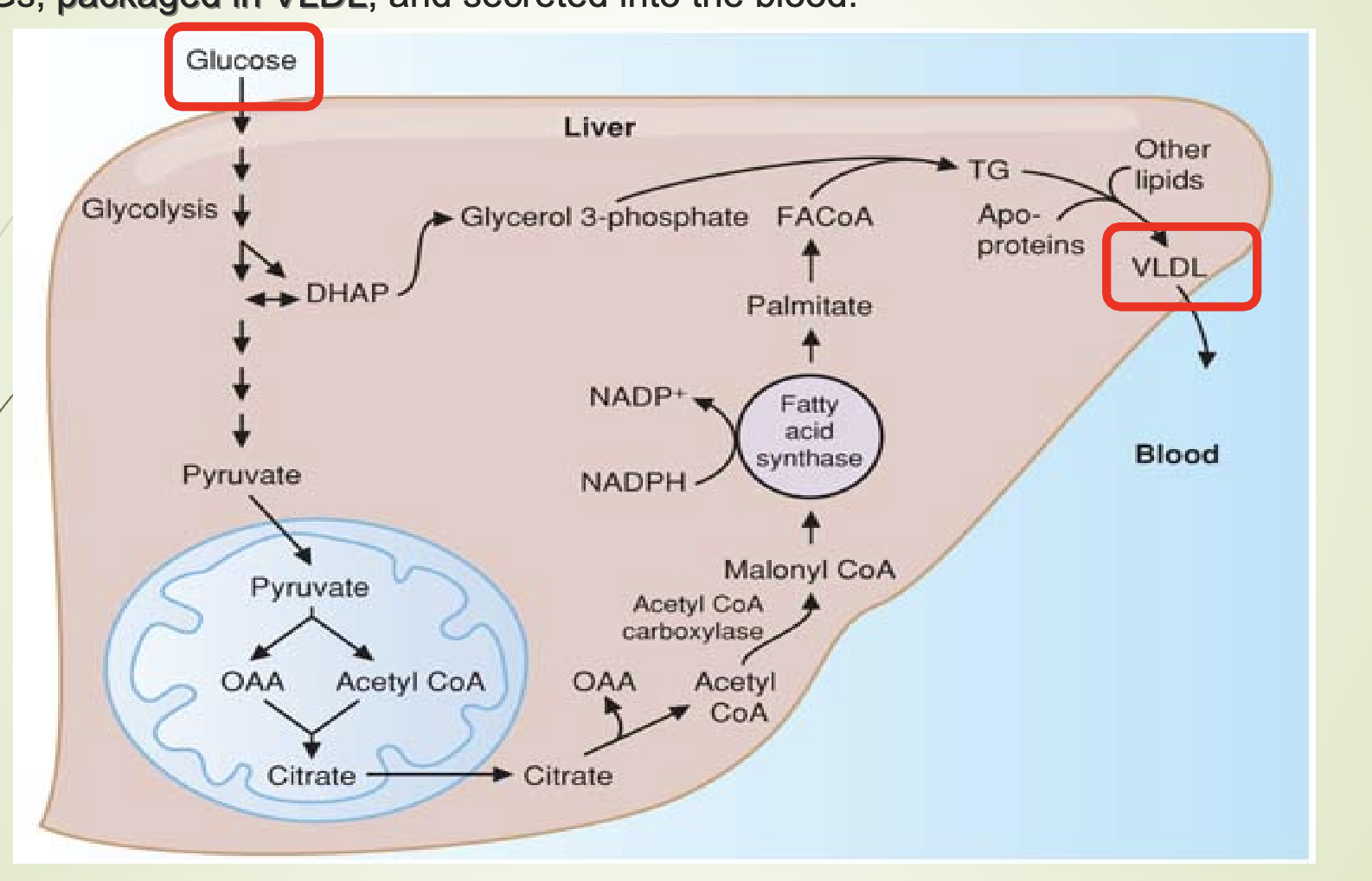
What is lipogenesis and where does it occur?
Lipogenesis = synthesis of TGs from glucose.
Occurs in the liver.
Fatty acids synthesized from glucose → converted to TGs → packaged into VLDL → secreted into blood.