Chemistry
5.0(1)
Card Sorting
1/25
Last updated 11:19 PM on 4/24/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
1
New cards
Isotopes
* atoms of a given element that differ in the number of neutrons and consequently in mass.
2
New cards
Because masses on the table are ***weighted averages*** of all known isotopes of the element of interest
Why are masses on the periodic table usually expressed as decimal numbers?
3
New cards
Metal ion \~ nonmetal ion
What ions are used in Binary ionic?
4
New cards
At least one ion is a polyatomic ion:
* Ammonium + negative ion\[nonmetal\]
* Positive ion\[metal\] + negative polyatomic ion
* Ammonium + negative polyatomic ion
* Ammonium + negative ion\[nonmetal\]
* Positive ion\[metal\] + negative polyatomic ion
* Ammonium + negative polyatomic ion
What ions are used in Ternary ionic?
5
New cards
Two nonmetal ions\[both negative\]
* prefixes must be used
* prefixes must be used
What ions are used in Binary molecular?
6
New cards
Hydrogen + nonmetal ions\[negative\]
* Hydroic acid
* Hydro
What ions are used in Binary acid?
7
New cards
Hydrogen + polyatomic ion
* -ic/-ide acid
*
What ions are used in Ternary acid?
8
New cards
Zinc\[+2\], Cadmium\[+2\] & Silver\[+1\]
what transition metals don’t require Roman Numerals?
9
New cards
* Acetate C2H302
* **Chlorate CIO3**
* **Chlorite CIO2**
* Dihydrogen Phosphate H2PO4
* Bicarbonate /Hydrogen Carbonate HCO3
* Hydrogen Sulfate HSO4
* **Nitrate NO3**
* **Nitrite NO2**
* Perchlorate ClO4
* **Chlorate CIO3**
* **Chlorite CIO2**
* Dihydrogen Phosphate H2PO4
* Bicarbonate /Hydrogen Carbonate HCO3
* Hydrogen Sulfate HSO4
* **Nitrate NO3**
* **Nitrite NO2**
* Perchlorate ClO4
What are some of the common polyatomic ions that have **-1 charge?**
10
New cards
* Carbonate CO3
* **Sulfate SO4**
* **Sulfite SO3**
* **Sulfate SO4**
* **Sulfite SO3**
What are some of the common polyatomic ions that have **-2 charge?**
11
New cards
* Phosphate PO4
* Phosphite PO3
* Phosphite PO3
What are some of the common polyatomic ions that have **-3 charge?**
12
New cards
Ammonium NH4
What are some of the common polyatomic ions that have **+1 charge?**
13
New cards
14
New cards
Law of Conservation of Mass
* Reactant + Reactant = Product
* Reactant + Reactant = Product
* The mass of all the reactants (the substances going into a reaction) must equal the mass of the products (the substances produced by the reaction).
* mass is neither created nor destroyed in any chemical reaction.
* mass is neither created nor destroyed in any chemical reaction.
15
New cards
**Boyle’s Law:**
* the volume of the gas varies **inversely** with pressure at a constant temperature
* PV
* the volume of the gas varies **inversely** with pressure at a constant temperature
* PV
What is the relationship of volume and pressure?
16
New cards
**Charles’ Law:**
* the volume of a fixed mass of a gas is **directly proportional** to its Kelvin temperature
* V/T
* the volume of a fixed mass of a gas is **directly proportional** to its Kelvin temperature
* V/T
What is the relationship of volume and temperature?
17
New cards
**Gay-Lussac’s Law:**
* pressure of a gas is **directly proportional** to the Kelvin temperature if the volume remains constant
* P/T
* pressure of a gas is **directly proportional** to the Kelvin temperature if the volume remains constant
* P/T
What is the relationship of pressure and temperature?
18
New cards
PV/T
formula for combined gas law
19
New cards
PV=nRT
Formula of Ideal Gas Law
20
New cards
* R = 8.314 LkPa/molK
* R = 0.08206 Latm/molK
* R = 0.08206 Latm/molK
What are the values for the ideal gas constant**[R]**?
21
New cards
273 Kelvin
What is the **Standard Temperature?**
22
New cards
* 1 atm
* 760 mmHg
* 101.3 kPa
* 14.7 psi
* 760 torr
* 760 mmHg
* 101.3 kPa
* 14.7 psi
* 760 torr
What is the **Standard Pressure?**
23
New cards
22\.4 L
What is the molar volume
24
New cards
6\.02 x 10^23 mol^(-1)
What is Avogadro’s constant
25
New cards
Dalton’s law of partial pressure
* P(total) = P1 + P2 + P3
* P(total) = P1 + P2 + P3
\
* states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases:
* states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases:
26
New cards
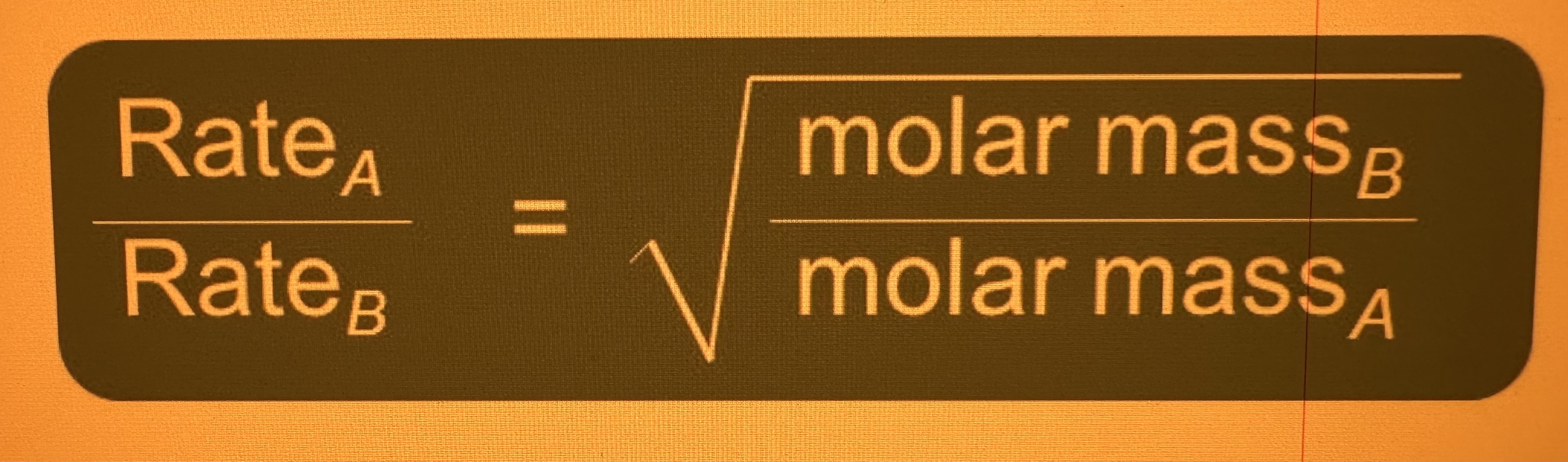
**Graham’s Law:**
* Rate(a)/Rate(b) = the square root of molar mass(b)/molar mass(a)
* Rate(a)/Rate(b) = the square root of molar mass(b)/molar mass(a)
* How do you get the ratio of effusion rates