3.2 - Mass number and isotopes
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
Mass no. and atomic no.
Mass no. (A)
Atomic (proton) no. (Z)
Isotopes
No. of protons and electrons stay the same but the no. of neutrons change
To find the no. of neutrons
Mass no. - atomic (proton) no.
Isotopes of elements will have the same chemical properties due to having the same electron configuration however different physical properties due to the different Mass number
Calculating Ar
Ar = total Mass of atoms/total no. Of atoms
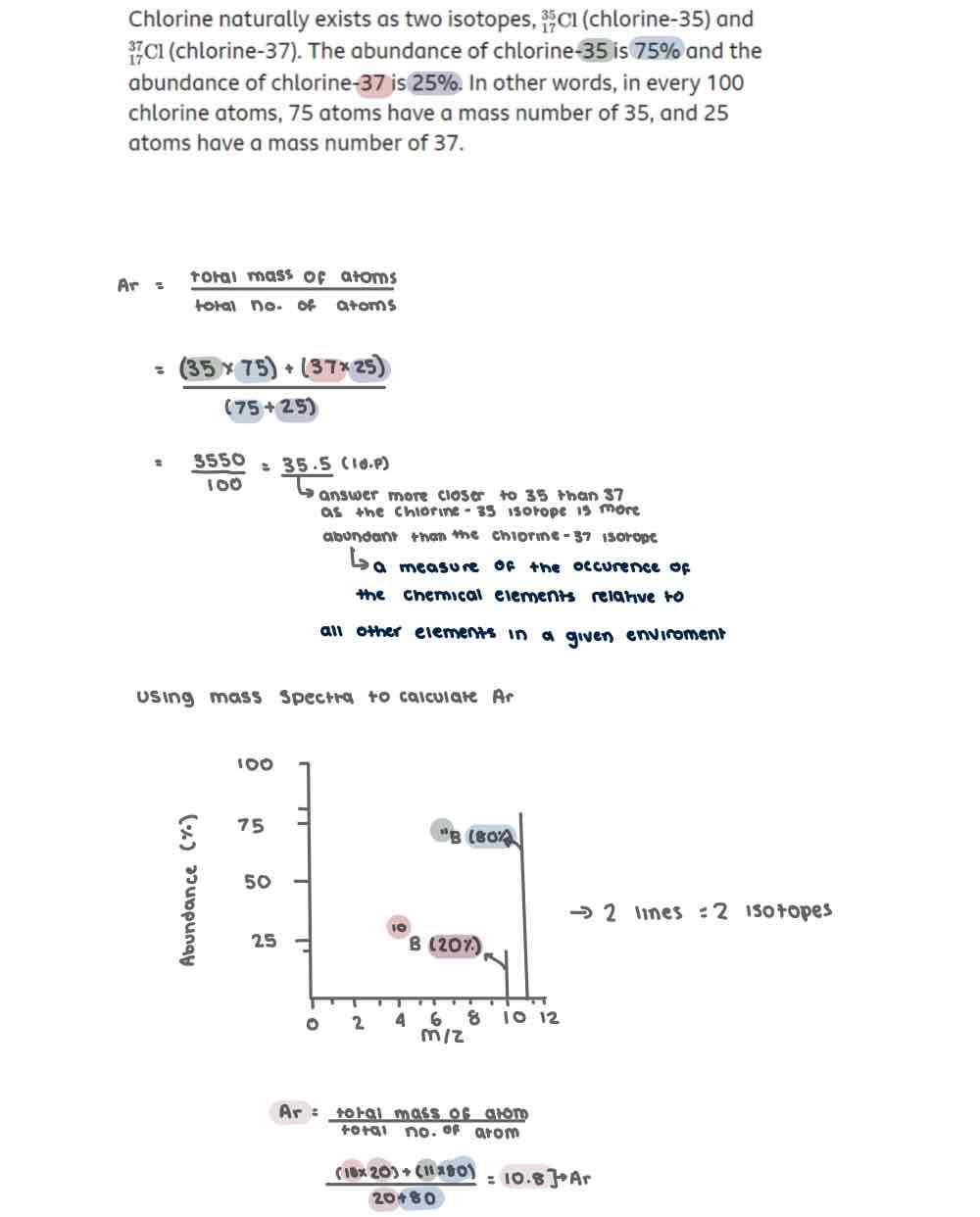
Ar (Relative Atomic Mass)
Average Mass of an element to 1/12 of the Mass of carbon 12 atom on a scale which carbon 12 has a mass of 12
Mr (molecular Mass)
Average Mass of a molecule relative to 1/12 of the Mass of carbon 12 atom (sum of Ar values for each atom within the molecule)
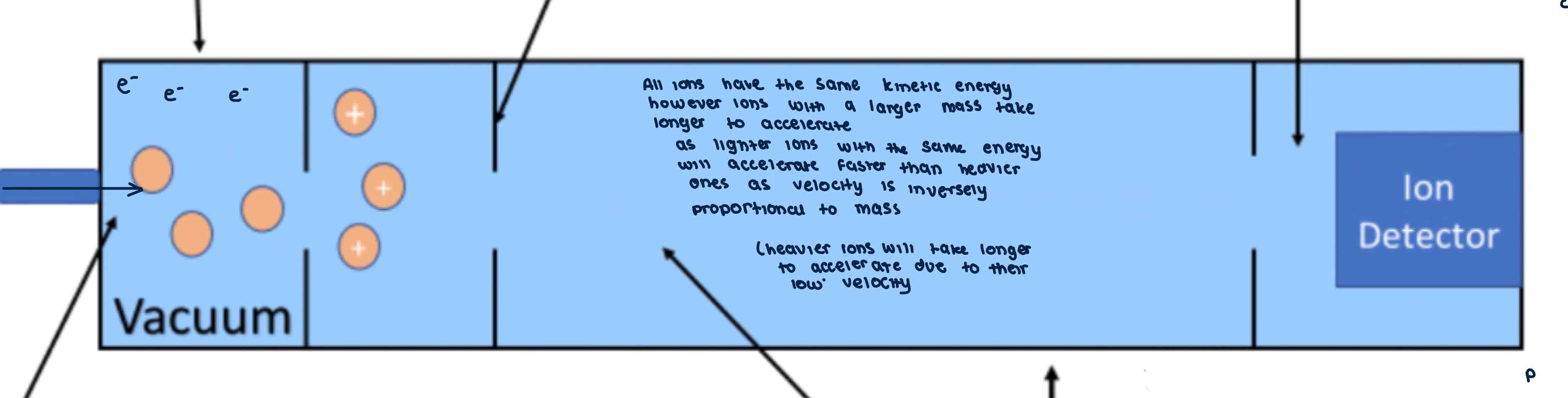
Time of flight mass spectrometry (TOF)
Mass spectrometry = instrument which can separate ionised/charged particles of different masses and determine amounts of each particle in a mixture
Vaporisation
Ionisation
Electric field
Ion drift
Detector
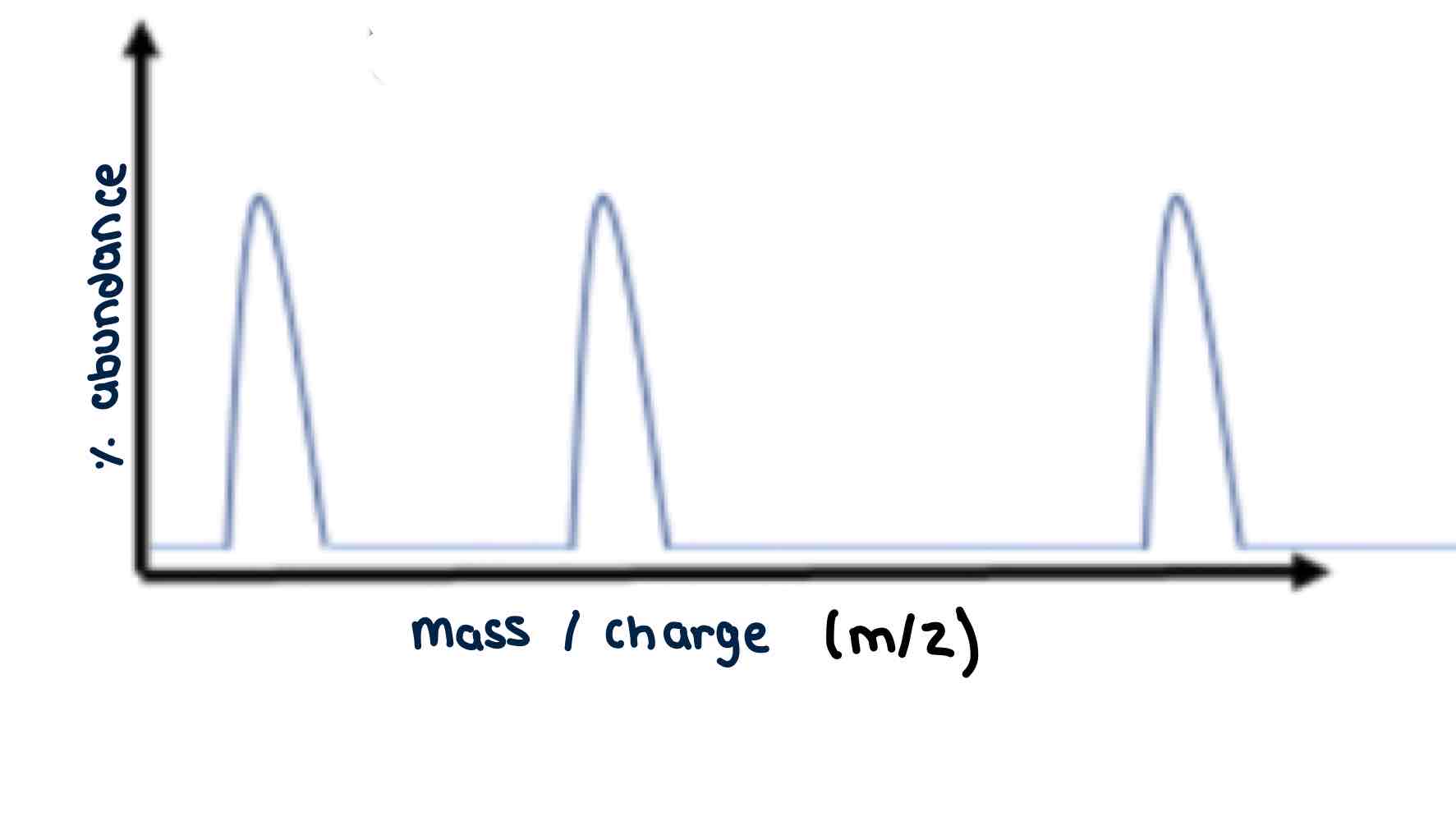
Mass spectrum
Vaporisation
Samples of unknown substance (element or compound) injected into a vacuum preventing the sample from being contaminated
Ionisation
2 types of ionisation
Electron impact ionisation
sample is ionised into +ive ions by firing e- at it (knocking outer e- from outer energy level)
This form of ionisation is used with elements and smaller compounds
Electro spray ionisation
sample is ionised into +ive ions by dissolving it in a solvent applying a voltage causing it to gain a H+ ion (when the H+ ion is gained the Mass of the sample increases by one) the solvent is then removed
This form of ionisation is used with large compounds stopping larger molecules fragmenting when ionised
Electric field
The electric field has a -ive charge to attract the +ive ions
The +ive ions are accelerated by the electric field
Lighter ions will accelerate faster than the heavier ones
Ion drift
The ions then drift with no electric field so ions are not deflected and just pass through (lighter ions drift faster)
Detector
The lighter ions hit the detector first then the heavier ions
The ions gains an e- when they hit the detector creating an electric current that can be detected
Mass spectrum
A Mass spectrum is generated using the charge ions
Is electron impact ionisation is used :
m/z = mass of isotope / charge
if Electrospray ionisation is used :
m/z ratio = (mass of isotope + Mass of H+) / charge
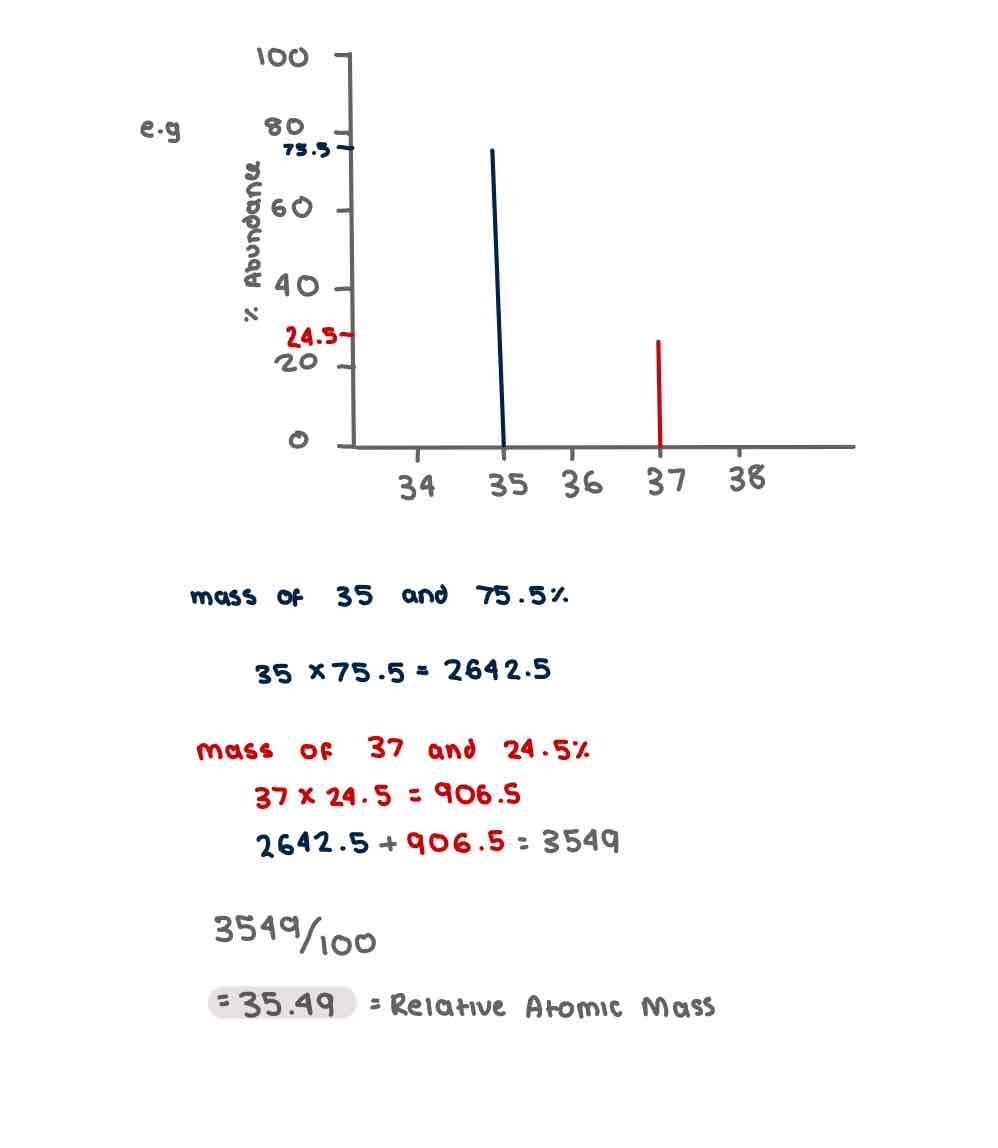
Calculating Relative Atomic Mass of an element using % abundance
Multiply the relative isotopic abundance (%) on the y axis by the m/z on the x axis for each isotope
Add up the total masses of each isotope amd divide them by 100
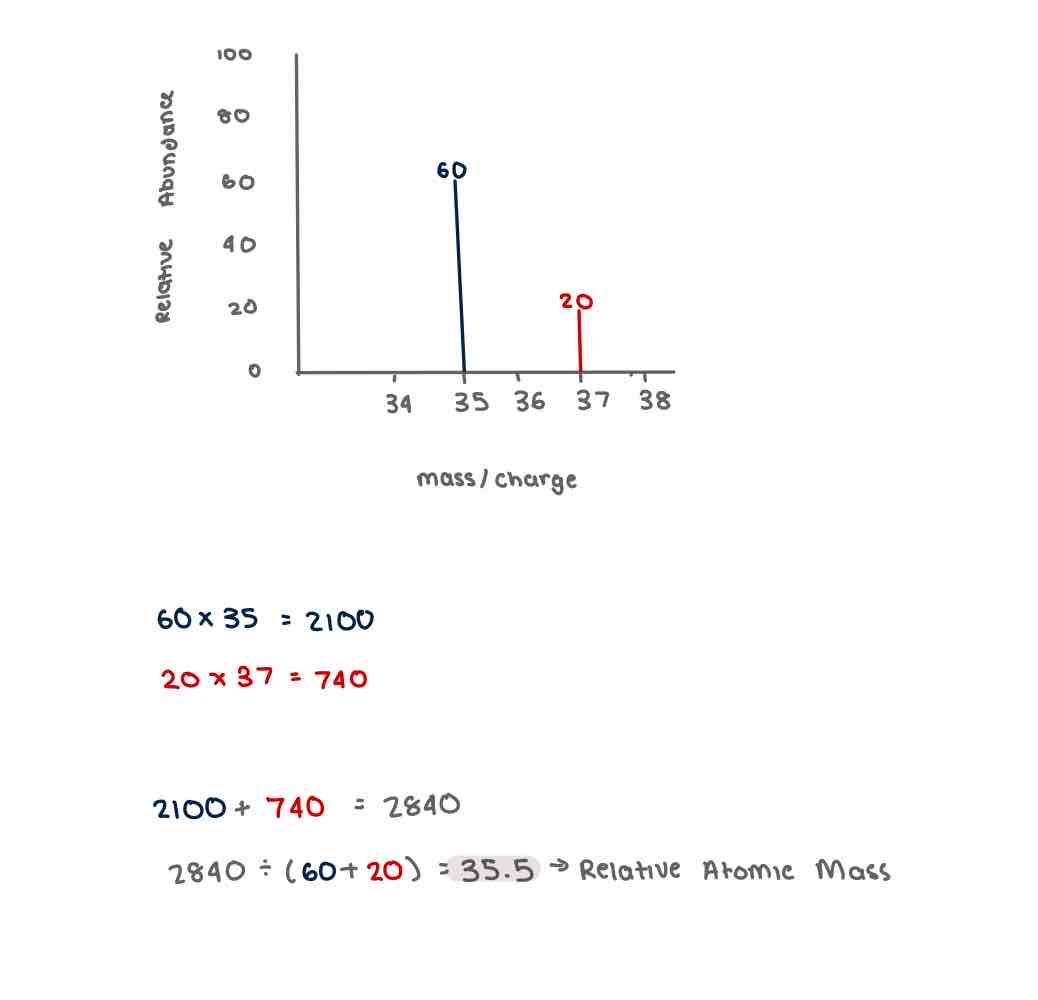
Calculating Relative Atomic Mass of am element using the unit of relative abundance (which is not given as a percentage)
If relative abundances are not given as a % divide by the total sum of the relative abundance
Problems with diatomic molecules
E.g Cl2
Molecular ions are formed
Cl’2+ → Cl + Cl+ ]→ it won’t be accelerated
Molecular ions may fragment (break up)
![<ul><li><p>E.g Cl2</p></li><li><p>Molecular ions are formed</p><p>Cl’2+ → Cl + Cl+ ]→ it won’t be accelerated</p></li><li><p>Molecular ions may fragment (break up)</p></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/f93a03a9-8786-45b1-b27c-cef2ac680d5c.jpeg)
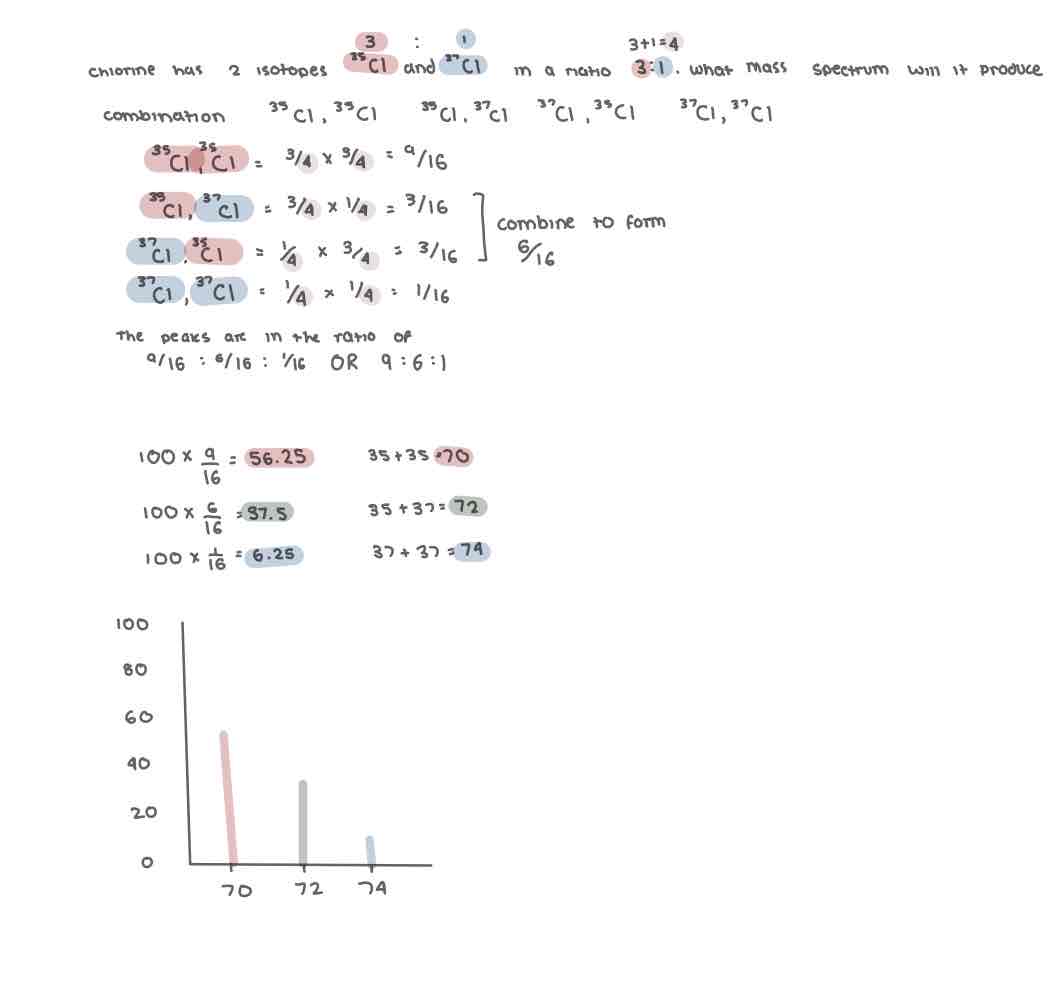
Predicting a mass spectrum of diatomic (x2) molecules with more than one isotope
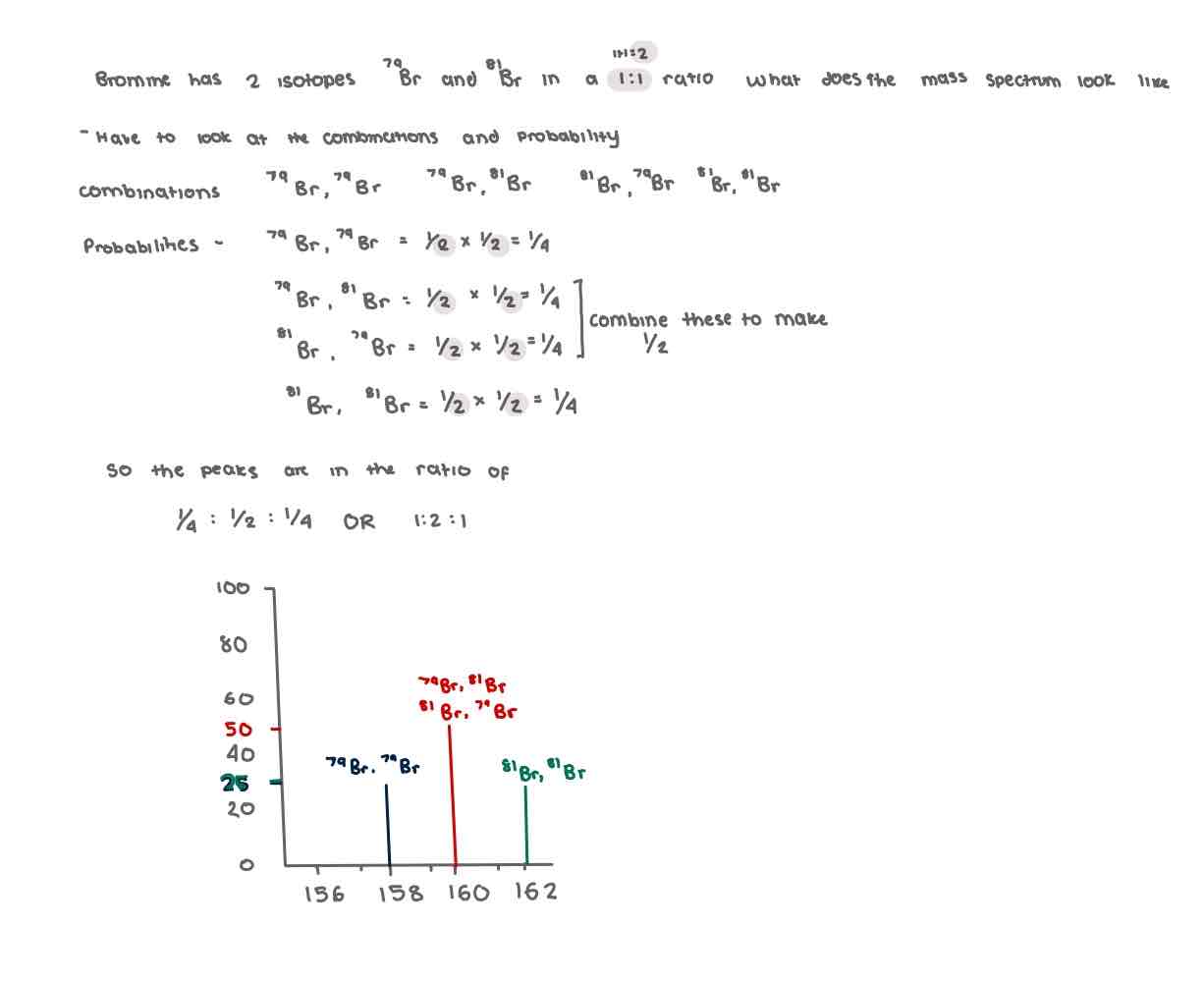
Predicting a mass spectrum of diatomic (x2) molecules with more than one isotope (2)