Science 3/28/23
5.0(4)
5.0(4)
Card Sorting
1/30
Earn XP
Description and Tags
Science test
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
1
New cards
Matter
Anything that has mass and takes up space
2
New cards
Solid
Matter that has a definite shape and volume
3
New cards
liquid
matter that does not have a definite shape but does have a definite volume
4
New cards
gas
matter that does not have a definite shape or volume
5
New cards
pure substance
made up of only one type of matter
6
New cards
homogeneous
every part of the substance has the same composition as every other part
7
New cards
mixture
made up of two or more different substances
8
New cards
mechanical mixtures
A mixture that does not have the same appearance throughout
9
New cards
heterogeneous
A mixture where can see the differences with the naked eye
10
New cards
heterogeneous mixtures
mechanical mixtures
11
New cards
Solutions
the same appearance throughout, but are made up of two or more substances
12
New cards
Particles
very small portions of matter
13
New cards
The particle theory of matter
a theory that describes matter.
14
New cards
Kinetic energy
the energy of movement.
15
New cards
Temperature
the measure of the average kinetic energy of the particles in a substance
16
New cards
Heat
the energy that transfers from a substance at a higher temperature to one at a lower temperature
17
New cards
A change of state
a change from one physical state of matter (solid, liquid, gas) to another
18
New cards
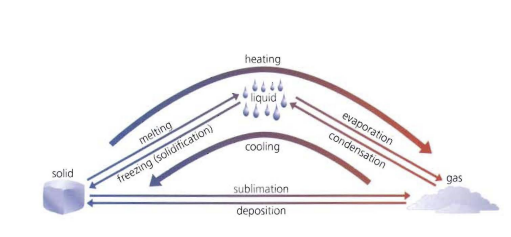
Look over this diagram
.
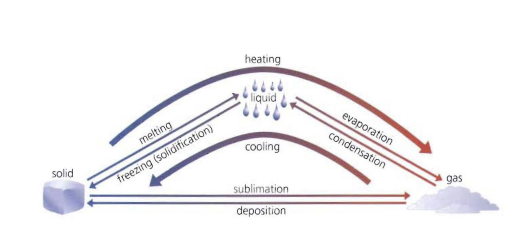
19
New cards
melting
The change of state from a solid to a liquid
20
New cards
evaporation
The change of state from liquid to gas
21
New cards
condensation
The change of state from gas to liquid
22
New cards
freezing/solidification
The change of state from liquid to solid
23
New cards
sublimation
The change of state from solid to gas
24
New cards
deposition
The change of state from gas to solid
25
New cards
Particle Theory of Matter Rule #1
All matter is made up of particles
26
New cards
Particle Theory of Matter Rule #2
All particles of one substance are identical
27
New cards
Particle Theory of Matter Rule #3
The particles of matter are in constant motion
28
New cards
Particle Theory of Matter Rule #4
Temperature affects the speed at which particles move
29
New cards
Particle Theory of Matter Rule #5
Particles have forces of attraction between them
30
New cards
Particle Theory of Matter Rule #6
There are spaces between particles
31
New cards
Mass
the amount of matter in an object