phy E3 radioactive decay
1/25
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
radioactive decay
unstable nuclide undergoes spontaneous random process, emitting alpha/beta particles and/or gamma rays
stable vs unstable isotopes
stable: no. of neutrons = no. of protons (for small nuclei) or neutrons > protons (for large nuclei). does not undergo radioactive decay. highest natural abundance among isotopes.
unified atomic mass unit
one twelfth of the rest mass of an unbound atom of carbon-12 in its nuclear and electronic ground state, having a value of 1.661×10-27kg
1u = 931.5 MeVc^-2
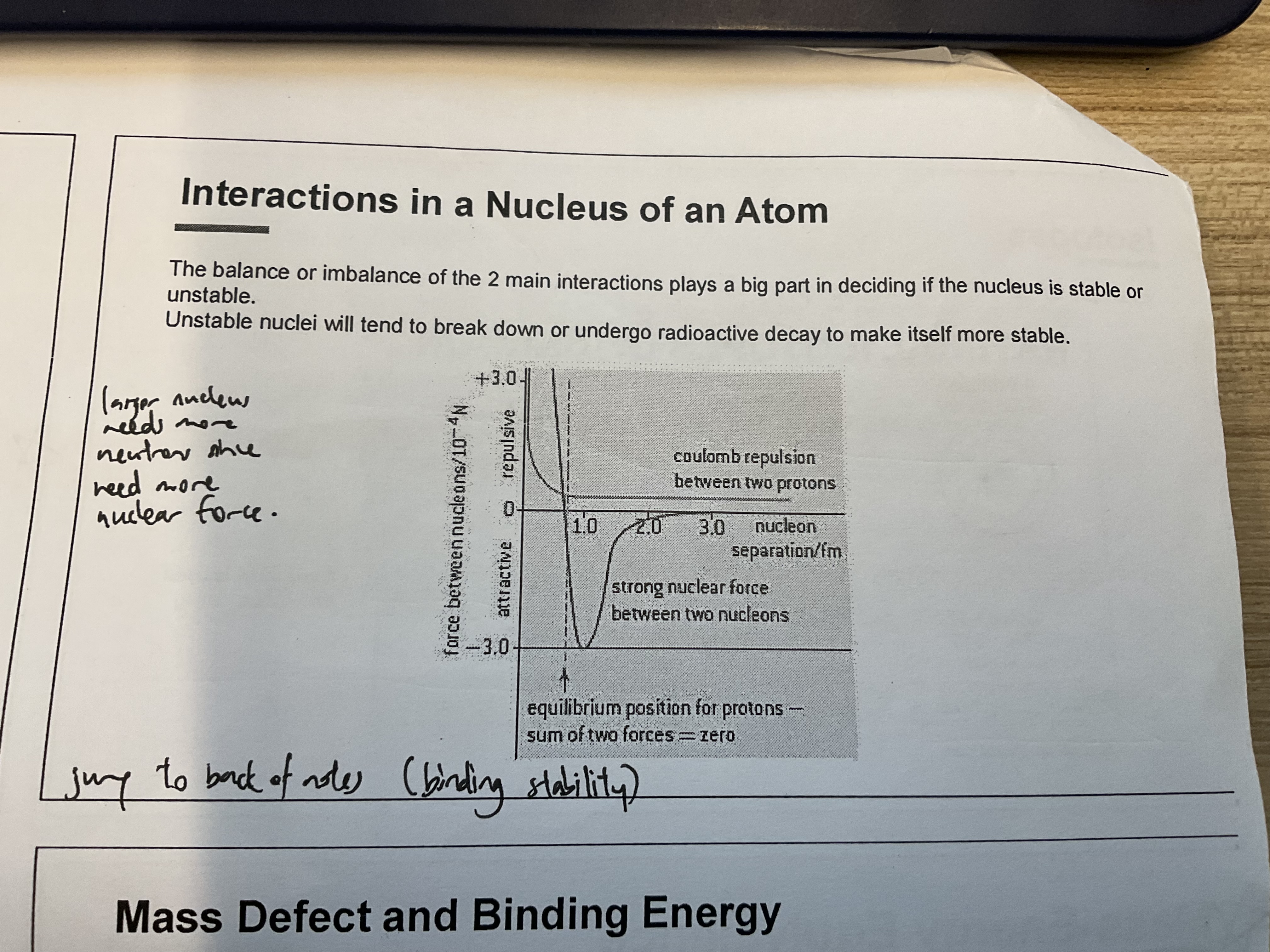
interactions in nucleus of atom
coulomb repulsion
protons all positive charge, repel each other
very significant because distance between protons is very small
strong nuclear force
strong short-ranged force (around 10-15m, only inside the nucleus)
nucleons (protons and neutrons) attract each other when near
small nuclei usually equal no. of protons and neutrons but large nuclei need more neutrons to hold together: coulomb repulsion long range force vs strong nuclear force short range → therefore more neutrons needed for stability in large nuclei
balance of coulomb repulsion and nuclear force keep the nucleus together
if imbalanced (too many/few neutrons), nuclei unstable, undergo radioactive decay to become more stable (decays until it falls in the band of stability)
mass defect
difference between total mass of constituents and the mass of nucleus
mass of nucleus < mass of constituents because mass was lost as energy during formation of nucleus (binding energy)
binding energy
the amount of energy released when nucleus is assembled from its constituent nucleons OR amount of energy supplied to separate nucleus into its constituent nucleons.
energy lost to form bond
energy gained (work done) to overcome coulomb repulsion
loss > gain → overall loss in energy (binding energy is lost)
BE = 1 – 2
the higher the BE per nucleon, the more stable the nucleus
when A>60, BE per nucleon is about 8MeV
fission/fusion is energetically feasible if BE per nucleon of products > reactants (ie products more stable)
spontaneous
radioactive decay is not triggered by external factors
random
radioactive decay is unpredictable, cannot determine exact moment of decay
why alpha/beta particles have circular motion when entering magnetic field?
alpha and beta particles are charged, by FLHR will have circular motion
beta particle radius of motion smaller because less mass
r = mvsinθ / Bq
gamma ray has no charge (EM radiation) so will be in straight line
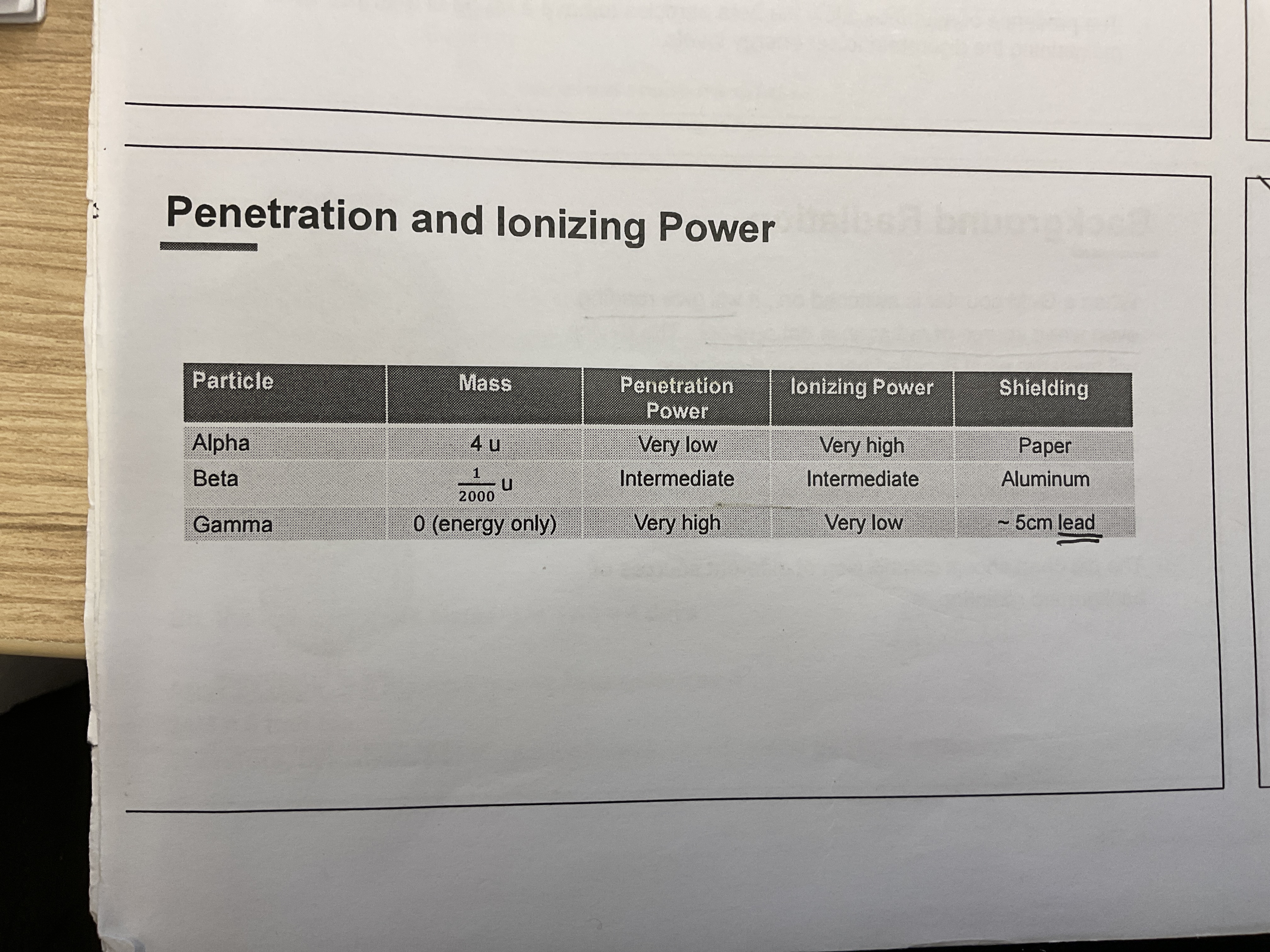
alpha particles
has 2 protons, 2 neutrons, net charge of +2
among emissions, alpha particle is the most stable, heaviest, least penetrating, highest ionising power
alpha decay most likely because alpha particle is most stable
alpha decay: mass - 4, charge - 2
beta particles
either electron (beta minus decay, charge -1. accompanied by anti-neutrino) or positron (beta positive decay, charge +1. accompanied by neutrino)
are emitted with continuous range of energy, large variation in KE. unexpected as it deviates from laws of conservation of energy and momentum → difference in energy/momentum is due to emitting neutrino/antineutrino
beta minus decay
when too many neutrons compared to protons
neutron changes to proton + electron + anti-neutrino
neutrino
explaining presence of neutrinos: electron emitted during beta decay expected to have KE of 156keV, but measured lower energy (and it was a continuous range of energy, not discrete) → particle that is difficult to detect is carrying off the energy and momentum (therefore conversation of energy/momentum still applies, can have range of energies while maintaining discrete nuclear energy levels)
neutrino has 0 charge, very small rest mass
beta positive decay
when too many protons compared to neutrons
proton converted to neutron, emits positron and neutrino
gamma rays
gamma rays are high-energy photons (EM waves)
unstable nucleus decays from excited state to lower state → photons emitted
nucleus may already have undergone beta decay and lost positrons, but still too excited (eg 12C → 12C is the same just that lose energy through gamma radiation to become stable)
comparison of the 3 particles
α decay most likely because alpha particle is most stable
alpha particles are highly ionising due to their charge and large mass. → Due to its ionising ability, an alpha particle has very short range as it loses its energy rapidly.
gamma rays are weakly ionising because they are electromagnetic waves and carry no charge and no rest mass. Hence, they have the highest penetrating power.
beta minus: neutron becomes proton, emits electron (negative) + antineutrino (no charge and no mass)
beta positive: proton becomes neutron, emits positron (positive) + neutrino (no charge and no mass)
antiparticle
same mass, different charge
position and electron
neutrino and anti-neutrino
geiger-muller counter
used to measure radioactive decay near to a source → so that α particles can be detected
setup
ionising chamber: metal cylinder filled with low pressure gas
thin mica window: allows radiation to enter
high voltage connected across casing of tube and central electrode
radiation ionises the gas → electrons/ions produced are drawn to electrodes → produces pulse of current → measure amount of radiation using counting circuit
α particles low penetration power, but still detected by GM counter because thin mica sheet can be penetrated
gamma rays low ionising power but still detected because the high voltage amplifies the signal
background radiation
even when no source of radiation, will measure background radiation that’s produced naturally from air, rocks, soil etc
background radiation can be measured over one-minute intervals
radioactive half life
time taken for half the number of unstable nuclei in a given sample to decay
decay constant λ
probability of decay of a nucleus per unit time → unit is s-1 or year-1
it is a constant that is unique to that particular nuclide
no. of decays in a short time: change in N = -λN X change in t
rate of decay (dN/dt) = -λN
law of radioactive decay
rate of decay decreases exponentially over time for a fixed sample,
no. of parent nuclei will decrease exponentially with time, rate of decrease depends on decay constant λ
N = N0e-λt
→ because the higher the initial no. of nuclei, the more decays there will be. but each decay decreases the no. of nuclei.
activity
(of a source) number of nuclei decaying per unit time → unit Bq
cloud chamber
vapour turns into droplets of liquid when radioactive particle travels through it
alpha particles form thickest lines, then beta particles
gamma ray does not have enough ionising power to form any lines
applications of radioactive decay
use isotopes of oxygen sulfur and carbon to determine chemical composition of different materials and surfaces
gamma-emitting tracers detect leaks in underground pipes
carbon dating using carbon-14 to estimate age of specimen
when specimen alive, keeps absorbing 14C
when dead, 14C decays (activity is the rate at which unstable atomic nuclei in a radioactive sample decay)
convert energy in MeV to J
energy in MeV x 10^6 × 1.6×10^-19