Unit 7: Metabolism and Energy Balance
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
102 Terms
what are the 3 possible fates for ingested macromolecules
fuel - metabolizes to provide energy
build - synthesis reactions for growth and maintenance of tissues
store - as glycogen (liver, skeletal muscles, or fat)
what are the 2 states metabolism is divided into? are they catabolic or anabolic
fed / absorptive state - anabolic products of digestion being absorbed and used for synthesis or stored
fasted / post absorptive state - catabolic, body taps into stores
what are 3 nutrient pools available for immediate use (mostly circulating in plasma)
glucose
free fatty acids
AAs
explain the enzymes that control direction of metabolism
Fed state:
metabolism is under the influence of insulin → enzyme activity for forward reaction inc
enzymes for glycogen breakdown are inhibited
net glycogen synthesis
Fasted-state:
metabolic under influence of glucagon, etc.
enzymes that break down glycogen are more active
enzymes for glycogen synthesis are inhibited
what is gluconeogenisis
producing glucose from lactate, glycerol, or AAs
usually done in the liver during the fasting state
triggered by glucagon or cortisol
what is glycogenisis
converting glucose to glycogen to be stored
usually done in liver and skeletal muscle during the fed state
triggered by insulin
what is the diff between glycogen and glucagon
Glycogen is a stored form of glucose found in the liver and muscles, used for quick energy.
Glucagon is a hormone released by the pancreas when blood sugar is low; it signals the liver to break down glycogen or make new glucose to raise blood sugar levels.
explain the nutrient storage depots of carbohydrates, fats, and proteins (and what happens in the TCA cycle) in the fed state
Fed State (high insulin, low glucagon)
The body stores nutrients:
Carbohydrates get stored as Glycogen:
Excess glucose is stored as glycogen in liver & muscle (glycogenesis)
Carbohydrates → Fat:
Glycogen stores are full → extra glucose is converted to fatty acids (FAs) → combined with glycerol → stored as triglycerides (TGs) in adipose tissue (lipogenesis).
Proteins → Body Proteins:
Proteogenisis
Excess AAs (not used for proteins) can be converted to pyruvate or acetyl-CoA where it’ll be turned to → FAs (fatty acids) → TGs (tri glycerides).
TCA Cycle: ATP production
Main hormone: Insulin promotes uptake & storage of glucose, AAs, and FAs
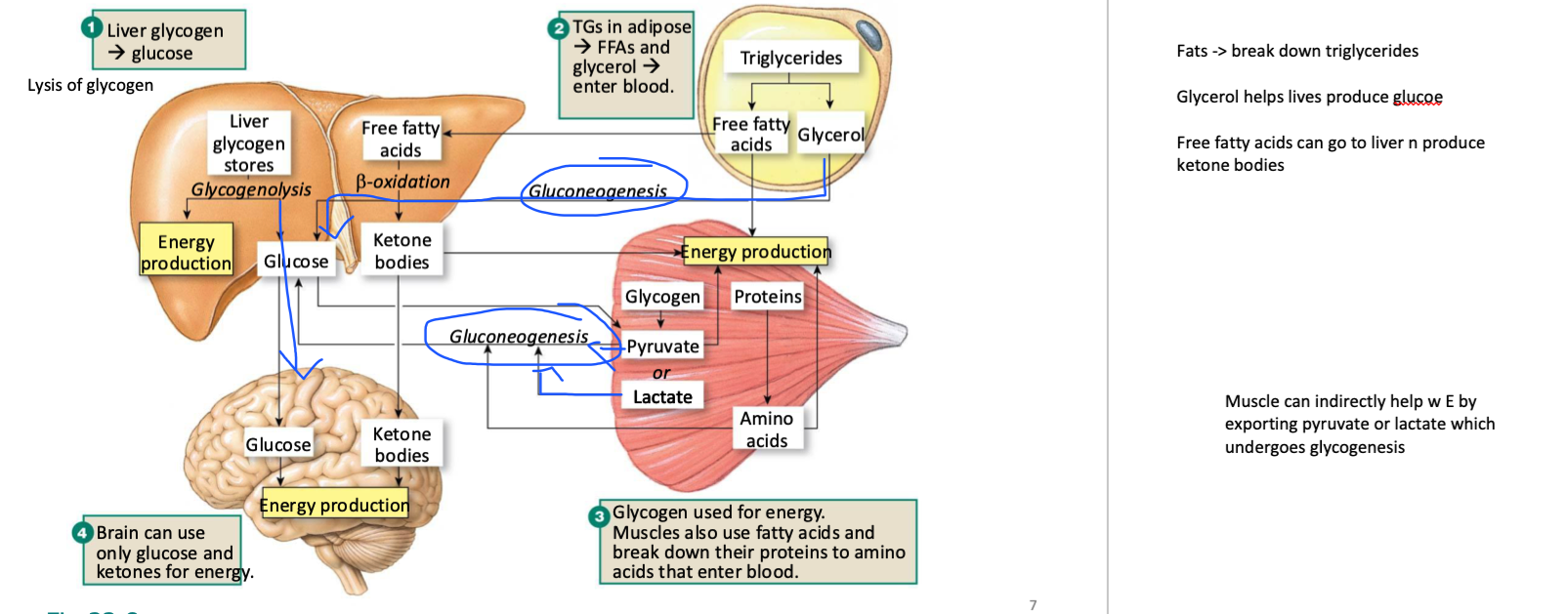
explain the nutrient storage depots of carbohydrates, fats, and proteins (and what happens in the TCA cycle) in the fasted state
Fasted State (low insulin, high glucagon)
The body mobilizes stores for energy:
Glycogen → Glucose: (Glycogenolysis)
glycogen in liver→ releases glucose into blood to maintain normal blood sugar.
glycogen in muscle → can NOT be exported as free glucose
can only be used by that muscle OR exported as pyruvate or lactate to the liver where it would undergo gluconeogenisis
Proteins → AAs → Glucose:
during extreme starvation
Muscle protein breakdown (proteolysis) to make AAs
liver converts them (via gluconeogenesis) into glucose.
OR they can be used within the muscle
Fats:
TGs in adipose tissue are broken down to make FAs + Glycerol:
Glycerol → exported to liver to undergo glueoneogenisis (prod glucose)
FAs → undergo beta oxidation (E prod)
make Acetyl-CoA
if fatty acids are exported to liver, liver uses it to prod keytone bodies from FAs (through ketogenisis)
FAs → Acetyl-CoA → Ketone Bodies: In prolonged fasting, liver uses FAs to make ketone bodies → alternative fuel for brain & tissues.
Main hormone: Glucagon promotes breakdown of glycogen, fat, and protein to maintain blood glucose & provide energy
what is beta oxidation
a metabolic process that breaks down fatty acids to produce energy (acetyl-CoA)
what are triglycerides broken down into during the fasted state?
into free fatty acids and glycerol (lipolysis)
then fatty acids to acetyl Co-A (beta-oxidation)
can muscle help with E prod? if so how
Muscle can indirectly help w E by breaking down glycogen into pyruvate or lactate and exporting it to the liver where it can undergo glycogenesis
what does the brain use for energy
glucose and ketones
when does ketogenesis occur
if lipolysis proceeds faster than acetyl CoA is being used in the TCA cycle (have excess CoA that is broken down into ketone bodies)
what does ketogenesis do
forms keytone bodies
can enter blood and serve as energy substrates for brain during times of starvation
know your starving bc fats are being broken down for energy faster than ATP is being produced for energy
what type of diet typically results in ketones synthesis
typically generated by low carb, high fat/protein diets (dont have carbs needed to undergo TCA cycle fast enough → body breaks down CoA to substitute)
why can ketones become dangerous in the body
certain ketone bodies are strong metabolic acids that can disrupt acid-base balance (called keto acidosis)
what is homeostatic eating
eating when energy fuels are depleted
not eating when energy fuels are sufficient
metabolically-driven eating
what is non-homeostatic eating and what does it involve
eating in the complete absence of hunger
eating despite large fat reserves
involves cognitive, reward, emotional factors
has neural parallels w addiction mechanisms
hedonic eating
what is hedonic eating
when food is consumed primarily for pleasure and enjoyment, rather than to satisfy hunger or nutritional needs
where are the “hunger”/”feeding” centre and “satiety” centres located
they are the 2 centers in the hypothalamus
what is the glucostatic theory
theory that food intake is regulated by glucose levels, monitored by centres in the hypothalamus
plasma glucose low → satiety centre (in hypo) is suppressed → feeding centre is dominant
what is the lipostatic theory? what resulted in the development of this theory
theory that signals from fat stores to brain modulates eating behaviour
discovery of protein hormone synthesized in white adipose tissue (meant to maintain a certain amount of fat)
protein hormone was called leptin (leptos = thin)
what does leptin do
tells the body to stop eating / when it has enough fat reserves
explain the story behind leptin and the ob/ob mouse
a spontaneous mut in rat colony in lab
mice were obese, voracious eaters
mut identified as ob/ob
protein product was leptin
mice homo for a mut in that gene were obese
another mut w similar phenotype was discovered to be mutation in leptin receptor
db/db
t/f: the body has more mechanisms to tell you to stop eating then to eat
true
what are signals from the gut that inc appetite
from stomach:
inc ghrelin
secreted by cells of empty stomach
what are signals from the gut that dec appetite in the stomach, upper small intestine, and lower small intestine/colon respectively
stomach:
inc stretch
inc acid (detected by acid-sensing ion channels)
upper small intestine:
inc CCK (in response to fat/protein in lumen)
inc glucose in lumen
lower small intestine/ colon
inc peptide YY (PYY) → inhibits relase of neuropeptide Y
GLP-1 (incretins)
both triggered by macronutrients in lumen and also neural reflex from upper small intestine
what is neuropeptide Y
a key neurotransmitter in stimulation of appetite
many hormones, neuropeptides, and products of adipocytes interact to influence appetite
Fat cells produce leptin, communicates above hypo feeding center (inhibits) to neuropeptide Y -> says to hypo to slow down food intake and also dec other neuro and hormones
what is hungry brain
larger animals (eg humans) have significant E stores - can go w out food for relatively long periods
BUT food restriction and fat depletion eventually lead to “hungry brain”
brain's inability to regulate appetite effectively
Good at defending the LOWER limits of fat supply (not depleting ALL of it and eating en mass when get close) → do not want to even get close to our lower limits so we get really hungry fast → trying not to eat even w prolonged hunger can result in hungry brain
with all our homeostatic mechanisms, why are humans obese
hungry brain
modern environment acts on higher brain regions to over-stimulate food intake
procuring food is no longer demanding or dangerous (easily accessible)
exceeding upper limits of body weight is no longer disadvantage in terms of preditor-prey
many obese humans become leptin-resistant
happens in many seasonal animals → elevated leptin doesn’t stop apetite in summer when food is abundant, but leptin sensitivity is restored in winter (making them want to eat less)
food is always readily availible so always in “summer” state
what were the first 2 reported cases of leptin deficiency in humans
2 severely obese first cousins from highly consanguineous (inbred) Pakistani family in UK
both born w homozygous frame-shift mut in lep (leptin) gene
5 other cases have been identified (from 4 other unrelated pakistani families)
how is metabolism regulated through the endocrine and NS respectively
endocrine - primary role
prod of endocrine pancreas
insulin/glucagon ratio
neural - regulation of food intake
endocrine pancreas is also innervated (autonomic responses)
what is the diff between glycogenolysis and glyconeogenesis
Glycogenolysis = break down glycogen for glucose (quick energy).
Gluconeogenesis = build new glucose from scratch (sustains blood sugar when glycogen is gone).
when do insulin levels increase? what are some processes that increase when insulin is increased
fed (absorptive state) triggers insulin release
energy production → glucolysis (breaking down glucose for E)
energy storage → glycogenesis (short-term) + lipogenesis (long-term)
protein synthesis (build things in body - eg muscle)
when do glucagon levels increase? what are some processes that increase when glucagon is increased
during the fasted (postabsorptive) state:
inc glyconeogenolysis (prod glucose for energy)
inc glyconeogenisis
inc ketogenesis
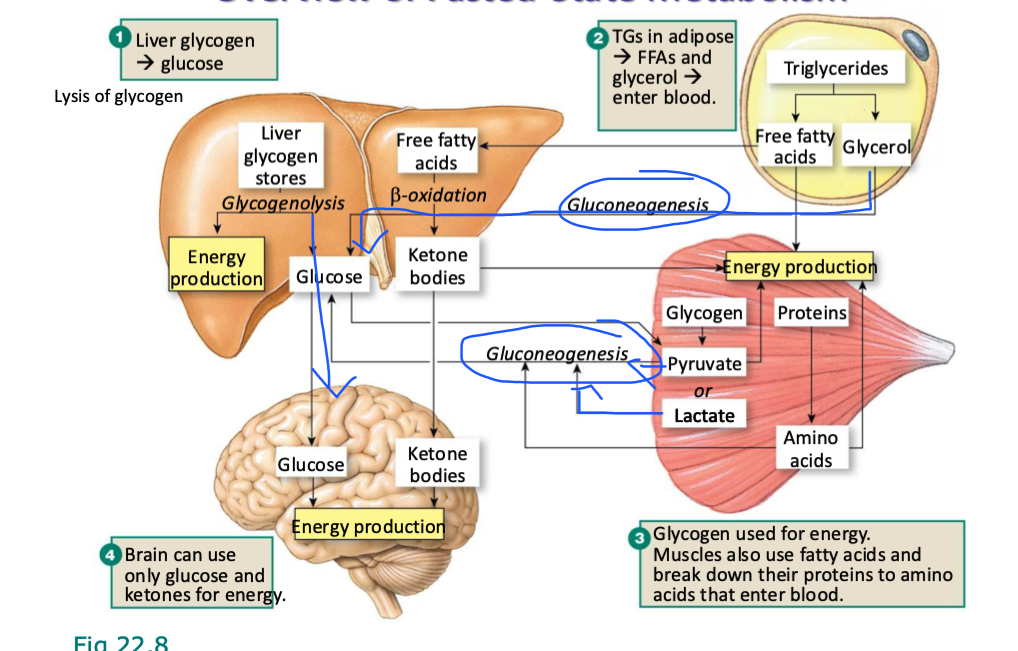
what happens to plasma insulin and glucagon levels immediately after a meal
plasma insulin inc immediately after meal
slowly dec after
plasma glucagon dec right after a meal
slowly inc after
what are the islets of langerhan → where are they located, what do they secrete, and how
lo
mix of…
beta cells making insulin
alpha cells making glucagon
D cells making somatostatin
majority are insulin producing
ductless granular cells (hormones)
t/f: the islets of langerhan are composed of exocrine tissue
F → endocrine
when are B-cells triggered and what does that do
releases insulin
inc plasma glucose & AAs
inc incretins
inc parasympathetic system
explain how and when B-cells are triggered
in fed state
eating → triggers, distension of GI tract, carbs in gut lumen, and nutrient digestion and absorption
Distension of GI tract
triggers stretch receptors
inc sensory neuron input to CNS (integrates)
inc parasymp response (rest and digest)
triggers B-cells
Carbs in gut lumen (eg glucose)
endocrine cells of small intestine sense and integrate info and trigger release of incretins (GLP-1 & GLP)
incretins trigger B-cells
nutrient digestion and absorption
inc plasma AAs & plasma glucose
triggers B-cells
(and plasma glucose inhibits alpha-cells)
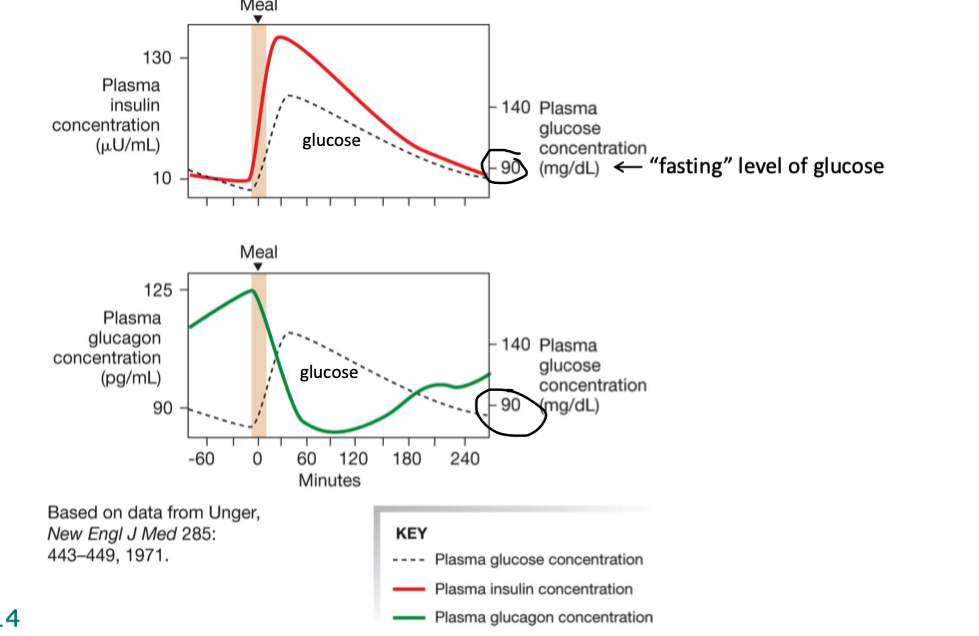
explain how B-cells do at rest
blood glucose is low (hence why at rest)
metabolism slows
glucose cannot enter B cells via GLUT2 transporters
dec ATP production
KATP channels (ATP-gated K channels on B cells) open, K leaves
cell is at resting mem pot
no insulin is released via exocytosis bc v-gated Ca channels remain closed
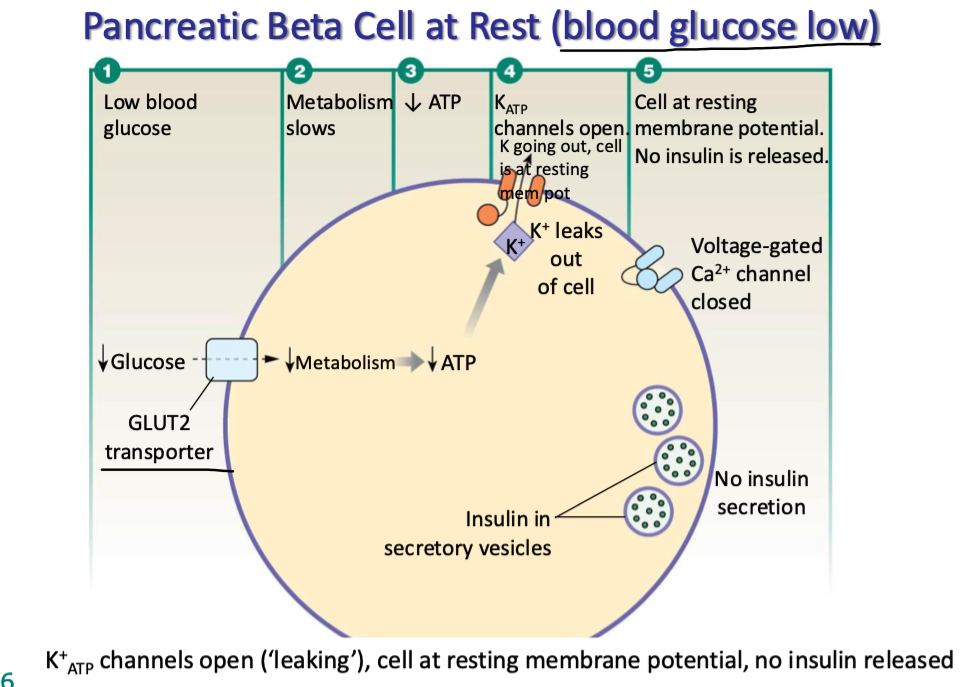
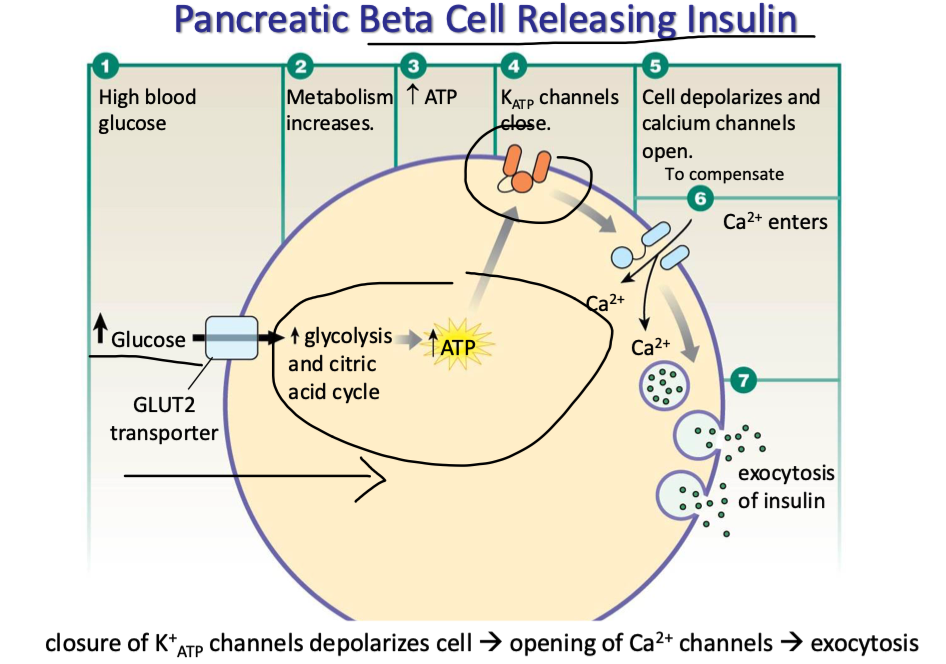
explain how pancreatic B-cells release insulin
high blood glucose
glucose enters B cells via GLUT2 transporters
metabolism inc
inc ATP prod
KATP channels close (K+ stuck in cell)
cell depolarizes → Ca channels open
triggers exocytosis of insulin
which cells does insulin usually act on? what does that do? (4 things)
primarily works on cells that express GLUT4 transporter → striated muscle, adipose tissue
and the liver.
stimulates inc of glucose transport into the aforementioned target cells (via GLUT4)
Inc glucose uptake from those various targets results in…
inc glycolysis (inc glucose metabolism to prod ATP)
inc glycogenesis (inc glycogen production for short-term energy storage)
inc lipogenesis (fat synthesis → for long-term energy storage)
inc protein synthesis (for building things within the body like within straited muscle)
BASICALLY insulin promotes enzyme activity that uses glucose.
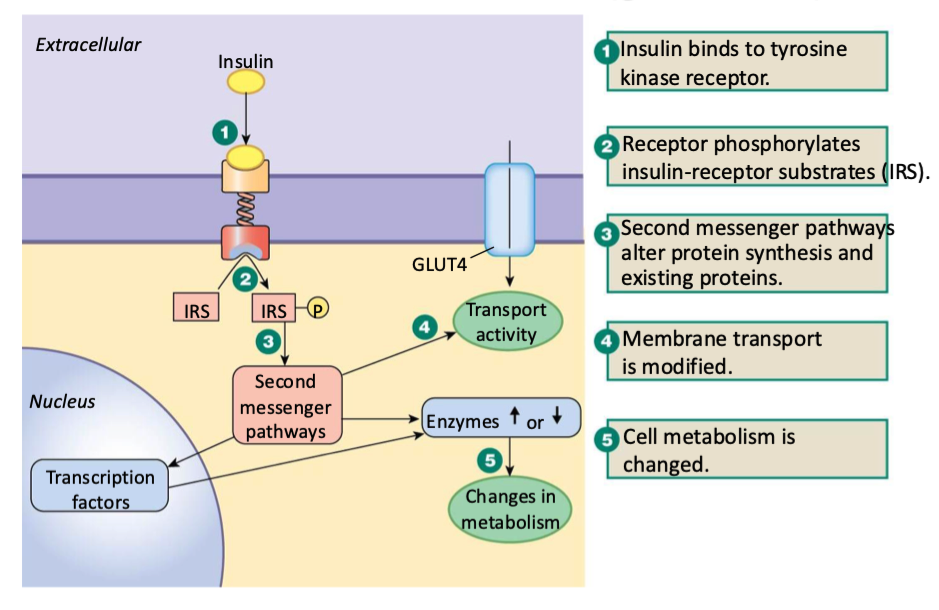
what is insulin’s general mechanism of action (how does it GENERALLY work on target cells)
binds to tyrosine kinase receptor
receptor phosphorylates insulin-receptor substrates (IRS) on inside of mem
2nd messenger pathways alter protein synthesis & existing proteins
mem transport is modified
cell metabolism is changed
change enzyme activity OR
change transcription factors which change enzyme activity
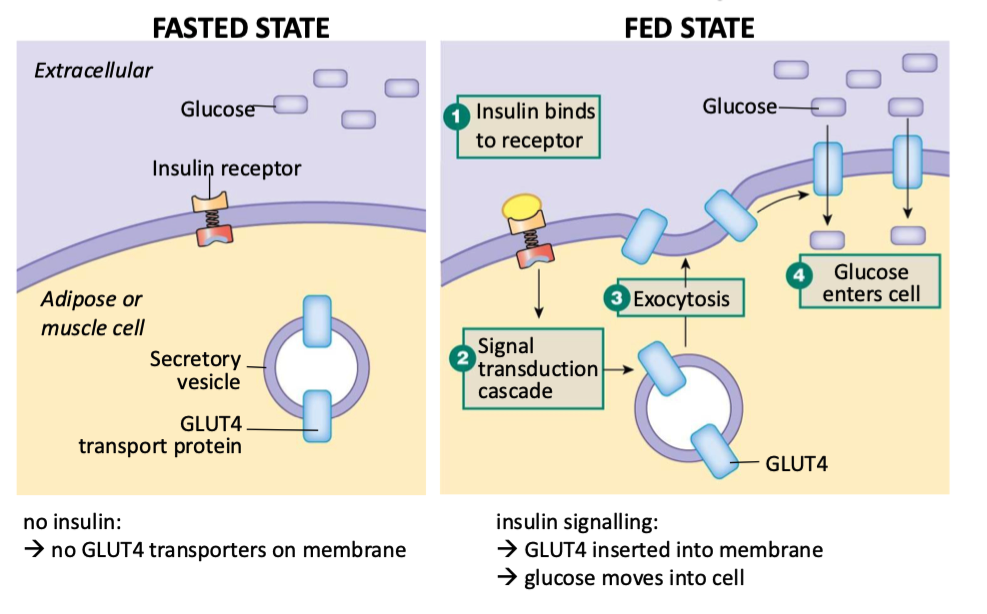
how does insulin effect muscle and adipose tissue in the fasted vs fed states
Fasted:
no insulin
insulin can’t bind to receptor
no GLUT4 transporters get put on mem
Fed:
insulin binds to receptor
triggers signal transduction cascade
GLUT4 inserted into mem via exocytosis
glucose moves into cell
explain how insulin effects hepatocytes
hepatocytes contain glucose transporters called GLUT2 transporters
fasted state:
blood glucose is low, insulin is low
hepatocytes make glucose and export it via GLUT2 transporters
fed state:
blood glucose is high, insulin is high
gradient favours glucose import via GLUT2
insulin signalling activates hexokinase
converts glucose to other things, keeps it low in cells
what is another term for glycolysis
glucose oxidation
is insulin catabolic or anabolic? explain why
anabolic
activates enzymes that enhance…
glycolysis (glucose oxidation)
glycogenesis
lipogenesis (conversion of excess glucose or AA to triglycerides)
AA utilization / protein synthesis
inhibits enzymes that enhance…
gluconeogenesis
glycogenolysis
lipolysis
beta oxidation of fatty acids
proteolysis
what is diabetes mellitus
group of diseases characterized by elevated blood glucose
what is hyperglycemia
high blood glucose
explain type 1 vs type 2 diabetes
type 1 → inadequate insulin secretion
type 2 → abnormal target cell responsiveness
what are the diffs in blood glucose levels in those w and w out diabetes after taking oral glucose. consider type 1 vs type 2
without → should see a spike in blood glucose levels that comes down and returns to normal after couple hours
with type 1 AND/OR 2 → diabetes (it doesn't matter in this case if it's type 1 or 2), their body would not be able to properly partake in its absorptive state actions, including breaking down glucose. So their blood glucose levels would spike and stay higher for significantly longer
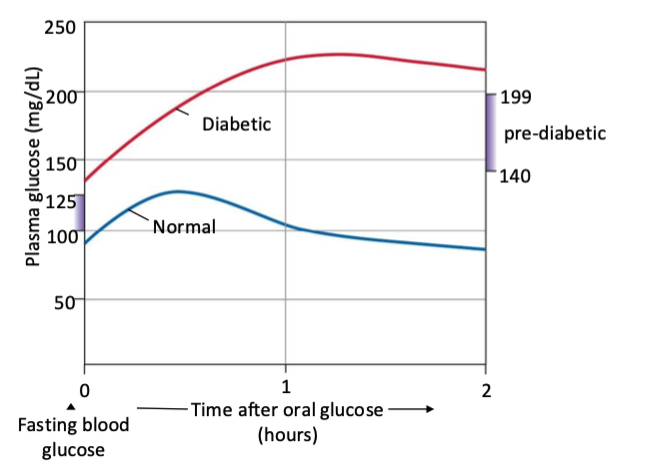
does insulin stay longer higher in type 1 or 2 diabetes
Both T1DM & T2DM show a delayed/abnormal glucose clearance, leading to prolonged hyperglycemia on an oral glucose challenge.
T1DM: Almost no insulin → glucose stays high.
T2DM: Delayed, insufficient insulin + insulin resistance → glucose stays high longer.
t/f: does diabetes have mostly harmless effects overall
false → it is metabolically catastrophic.
which type of diabetes is more common
type 2 (insulin resistance) → accounts for around 90% of diabetes
was once celled “mature onset” diabetes vs juvenile
t/f: type 2 diabetes can be coupled with low, normal, or high insulin secretion and have approx the same effects across
true
what are some defining physical characteristics of type 2 diabetes
acute symptoms are not as severe as type 1 BUT metabolism is not norma'l
atherosclerosis (buildup of fats, cholesterol and other substances in and on the artery walls) and hypertension often occur tg
typically in conjunction w obesity
metabolic syndrome

what is it called if you have at least 3 of these
metabolic syndrome
what is another member of the secretin family of peptides other than secretin, GIP, and GLP-1)
glucagon
what does glucagon antagonize
the effects of insulin
what is glucagon’s main target
the liver
what is glucagon’s main function? how much glucose is broken down at what times by what
to prevent hypoglycemia (low blood sugar)
during overnight fast, ~75% of glucose from liver comes from glycogenolysis (first step)
25% from glycogenolysis ~25% from gluconeogenesis (second step)
where is the adrenal gland located
on the kidneys
what does the adrenal gland produce
catecholamines - mostly epinephrine (adrenaline) → medulla is main source
explain the hypothalamic-pituitary-adrenal axis (with target organs and effects)
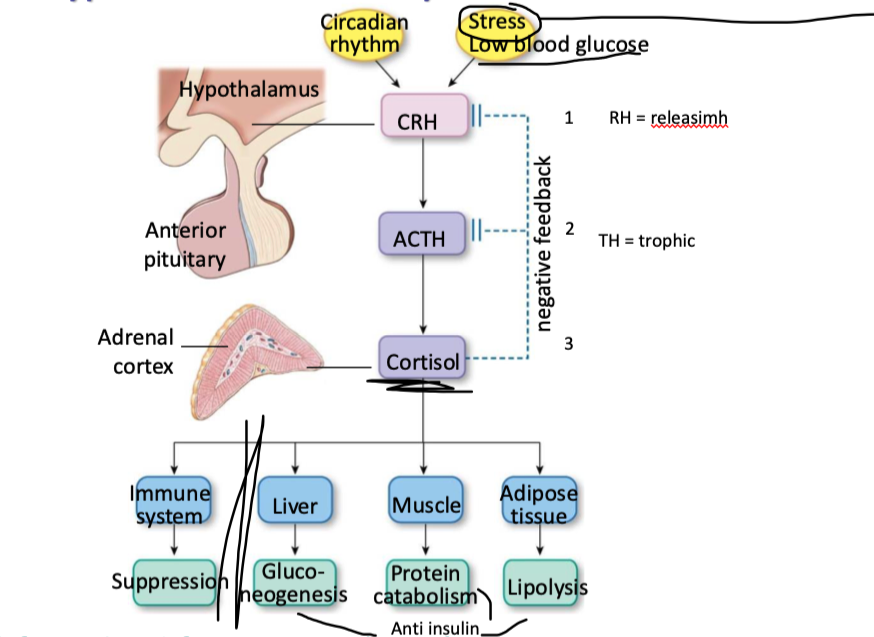
what are glucocorticoids
a type of steroid hormone produced by the adrenal glands, or synthetically created, that play a crucial role in regulating various bodily functions, including metabolism and the immune response
which cells have receptors for glucocorticoids
all nucleated cells
what kinds of effects do glucocorticoids have
mostly longer-term (genomic) effects → bc steroid
inc expression of enzymes
inc expression of receptors for other regulatory hormones
what are the 2 general effects of glucocorticoids
prevention of hypoglycemia
adipose → lipolysis → FAs for energy, glycerol
muscle → proteolysis → AAs
liver → gluconeogenesis
permissive for full effects of glucagon and epinephrine
suppress immune response
what did Hans Selye find out ab stress
developed concept that wide variety of “stressors” caused generic responses…
adrenal hypertrophy (bc trophic hormone was high)
atrophy of thymus / lymph nodes
GI ulcers
failure to cope w / adapt to stresses caused diseases of adaptation (ulcers, hypertension, etc)
led to many hypothalamic-pituitary axis breakthroughs
explain epinephrine in the fight or flight response
quick response
norepinephrine from sympathetic post-ganglionic neurons
epinephrine from adrenal medulla
rapid effects but short half-life (~2 mins)
metabolic effects: mobilize energy sources
dec insulin release, inc glucagon release
adipose → lipolysis → FAs for E, glycerol
muscle → glycogenolysis
liver → glycogenolysis, gluconogenisis
effects similar to glucagon but receptors expressed on broader range of target cells
tips balance to cranking up metabolism, breaking down fats, muscle, etc
what are 2 cortisol pathologies? explain them
hypercortisolism (Cushing’s syndrome, high cort)
primary → cortisol-secreting adrenal tumors (not regulated by ACTH)
secondary → pituitary tumor that over-secretes ACTH
iatrogenic → secondary to cortisol therapy for other conditions
hypocortisolism
primary → adrenal insufficiency (addison’s diseae)
adrenal gland develops abnormally
can b bc muts in key steroidogenic enzymes
congenital (born w) adrenal hyperplasia → enlarged adrenal gland
adrenal gland damaged / distroyed (autoimmute)
secondary → lack of ACTH
how do glucagon, epinephrine, and cortisol work on blood glucose
synergistically
which hormones act the fed state
only insulin
which hormones act in fasted state
epinephrine, glucagon, cortisol
where can gluconeogenesis occur
ONLY EVER OCCURS in liver
what do epinephrine, glycagon, and cortisol do in the fasted state (check this slide later)
epinephrine:
all liver functions in fasted state
glycogenolysis (in liver)
gluconeogenesis (only ever happens in liver)
glycogenolysis (in muscle)
lipolysis
can be used by muscle or go to liver
glucagon:
can only act on liver so has 3 options
glycogenolysis (in liver)
ketogenisis
proteolysis
lipolysis
gluconeogenesis (only ever in liver)
what is thyroid homrone classified as
an amino acid derivative (from tyrosine, contains iodine)
only known use of iodine in the body
what is thyroid hormone’s mechanism of action similar to
steroids → lipophilic, travels in circulation bound to thyroid-binding glubulin
binds to nuclear receptor
what is thyroid hormone’s main circulating form and its most active form? how does it switch btwn the 2
T4 - main circulating form
T5 - most active form, converted w in target cell by deiodinases (removing iodine)
what are some actions of thyroid hormone (what does it do)
essential for normal growth / development, especially NS
thyroid hormone levels checked in all newborns in canada
not essential in adults but affects quality of life
main fx → provide substrates for oxidative metabolism
inc O2 consumption and generation of heat (thermogenesis) in most tissues (maintains basal metabolic rate)
inc activity of Na/K pump
interacts w other hormones to modulate carb, protein, and lipid metabolism
what is hypothyroidism? what are some of its symptoms and its main cause
low O2 consumption, dec metabolic rate (cold intolerant)
neurological effects, fatigue
effects on skin, nails, hair
most common cause of hypothyroidism
iodine deficiency → enlarged thyroid gland → called “goitre”
what is hyperthyroidism? what are some of its symptoms and its main cause
inc o2 consumption, inc heat production (heat intolerant)
muscle weakness (protein catabolism/breakdown)
neurological, cardiac effects
exophthalmos (protruding eyes)
most common cause:
Graves disease → autoantibodies that resemble TSH overstimulate thyroid gland (not subject to - feedback regulation)
t/f: growth hormone can only effect target cells directly. explain
false → effects both direct and indirect
direct:
target cells that express GH receptor
indirect:
mediated by insulin-like growth factors (IGFs = somadomedins) prod by liver or target cell themselves
what are some metabolic actions of growth hormone
carbohydrate - indirect effects lead to inc plasma glucose
fats - inc lipolysis, inc oxidation
catabolic w respect to CHOs and fat “anti-insulin” effects
protein - inc AA uptake, inc protein synthesis, dec oxidation for E
anabolic w respect to proteins (pro insulin)
what are some growth actions of growth hormone
inc cartilage, bone, and muscle growth
explain why a deficiency in growth hormone could occur and what a common thing associated w it
due to GH hyposecretion and GH-receptor muts
dwarfism (though GH issues are not common cause)
what are some common things associated w excess growth hormone
depends on whether excess GH happens before or after closure of growth plates
before → gigantism
after → acromegaly
what does most Ca in the body do
composes the extracellular matrix of bones and teeth (99% of it in the body does this)
bone resevoir is main source of Ca but very little of it is free for exchange
what do osteoblasts do
lay down Ca-PO4 (build extracellular matrix of bones)
what do osteoclasts do
secrete enzymes / H+ that dissolve mineral matrix → resorption (incorporate Ca in matrix into bone)
what drives bone resorption (note: not reabsorption) and what does that do
parathyroid hormone and vitamin D
inc osteoclast activity relative to osteoblasts
how much ingested Ca is absorbed and how
about 1.3 absorbed → through transcellular and paracellular routes
how is Ca expelled out of body
primarily via kidneys → freely filtered, most reabsorbed
hormonally controlled reabsorption at distal nephron only
what is vitamin D
a family of fat-soluble vitamins → derivatives of cholesterol
get from plant/animal sources
UV rays from sun activates the precursor stored in our skin
liver adds hydroxy
kidney adds hydroxy
now works
what is 1-25-dyhydroxy cholecalciferol
Vitamin D
what is calcitriol
vitamin D
explain hwo Ca homeostasis is regulated
parathyroid hormone
released in response to hypocalcemia
drived release of V D3
Vitamin D3
released in response to PTH after being activated
tg they:
inc Ca absorption form intestine
inc Ca reabsorption in distal nephron (kidney)
inc resprotion from bone (inc osteoclast)