Chapter 7 - Extent Of Chemical Reactions
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
Closed System
Matter cannot be exchanged and energy can be exchanged with surroundings
e.g. A steam radiator only exhales heat (energy), whilst water is held within (matter)
Open System
Energy and matter is exchanged with surroundings
e.g. Boiling pot with water - heat (energy) and water (matter) is being exchanged
Non-reversible/Irreversible reactions
Products cannot be converted back into reactants (e.g. combustion reactions)
How reversible arrows are depicted
With double arrow
The collisions between particles in products can reform reactants
Examples of reversible batteries
Recchargeable batteries
Reversible Reactions Reaching Equilibrium
The rate at which the reactants keep turning into products = rate at which products keep turning into reactants
Rate of forward reaction = rate of reverse reaction
Concentrations of all products and reactants remain constant
Reversibility
If endothermic reaction (larger activation energy), reverse is exothermic (smaller activation energy) (and vice versa)
Dynamic State Of Equilibrium
Forward and reverse reactions always continue to occur (in equilibrium may halt or continue)
Reaction is incomplete (reactants and products are all in the mixture)
Bonds are constantly being broken and new bonds are being formed (reactants are turning into products and vice versa)
Occurs only in a closed system
Example
No further change between 8 seconds - 3hrs (dynamic equilibrium)
moving, but balanced - reaction hasn’t halted

Extent Of The Reaction
How far the reversible reaction has been converted into products/how much product is formed when the system reaches equilibrium
Is expressed as a fraction of the reactants that have been converted into products
Is from 0-1 (no products - full conversion to producst)
What Does Extent Of Reaction Not Indicate
Speed/rate of reaction
Can range from being fast - slow (not directly related to extent of reaction)
Example Of Extent Of Reaction (Unclear)
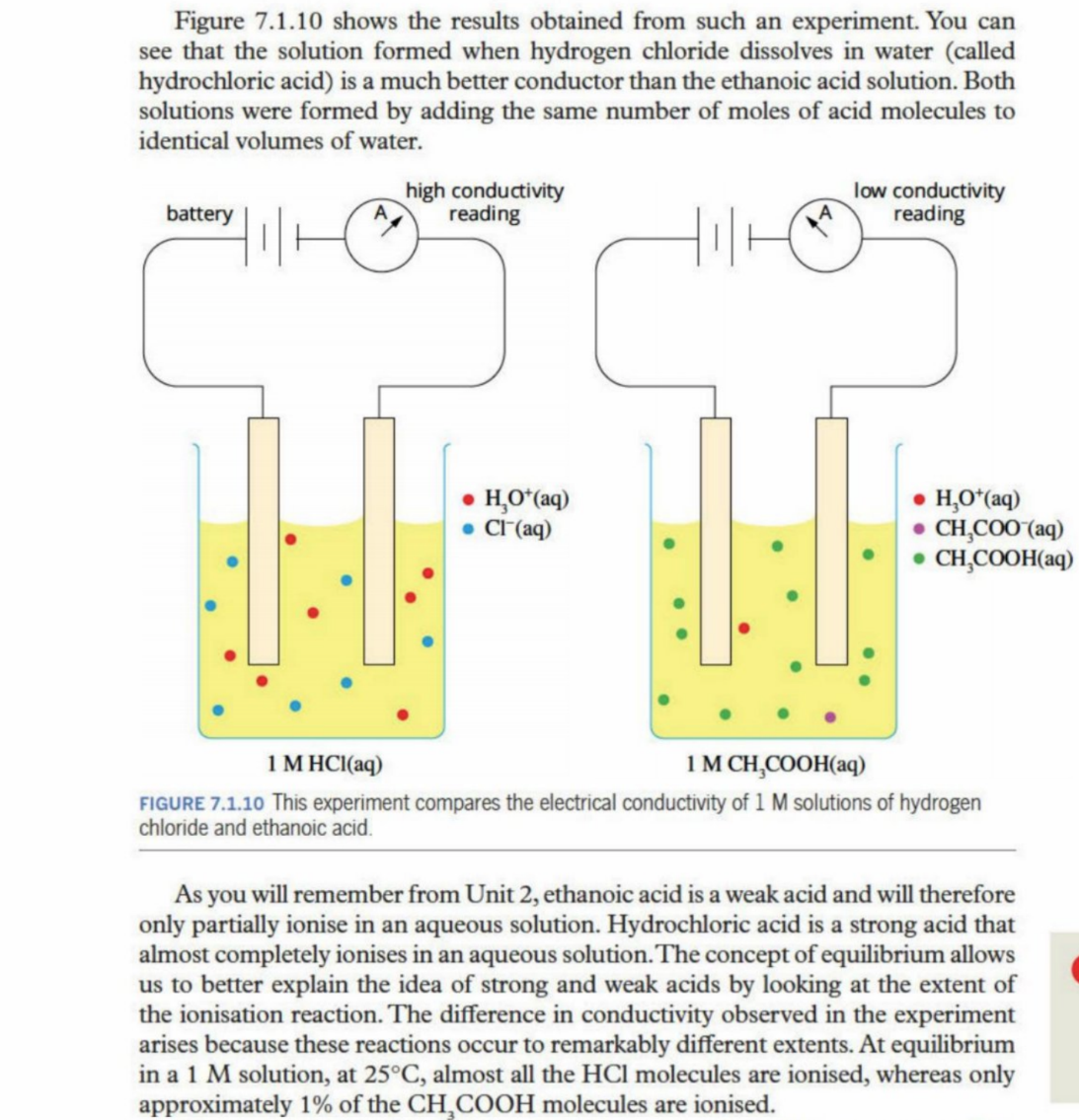
Example Of Concentration Graph
maybe incorrect - 6 seconds is when equilibrium is fully acheived

Equilibrium Law
It states if more products are being produced, more reactants are being produced, or if rate of production is equal
Equation for Equilibrium Law
Productscoefficients/Reactantscoefficients
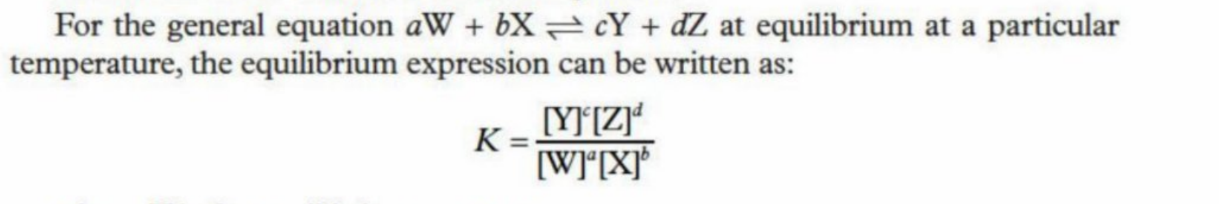
Explaining Law
Productscoefficients /Reactantscoefficients
If there are more than one product or reactants, you must multiply them together
K is the equilibrium constant (Kc or Kp)
When multiplying products or reactants
Are in variable form - e.g. “x” or “M”
This is for their units - deciding what unit output is at the end, not the actual concentration itself
E.g - Finding end unit
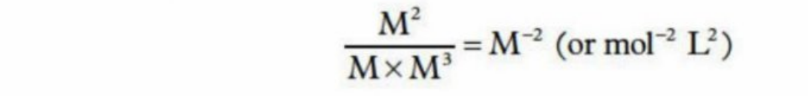
Reaction Quotient
Same as the equilibrium law, but is compared with “Q”
If K>Q, then more products are formed (shifted to the right)
If K<Q, then more reactants are formed (Shifted to the left)
If K=Q, then products and reactants are equal (equilibrium)
Q (Where reaction is at now), K (Where reaction should be) - theorised value
K vs Q Relation
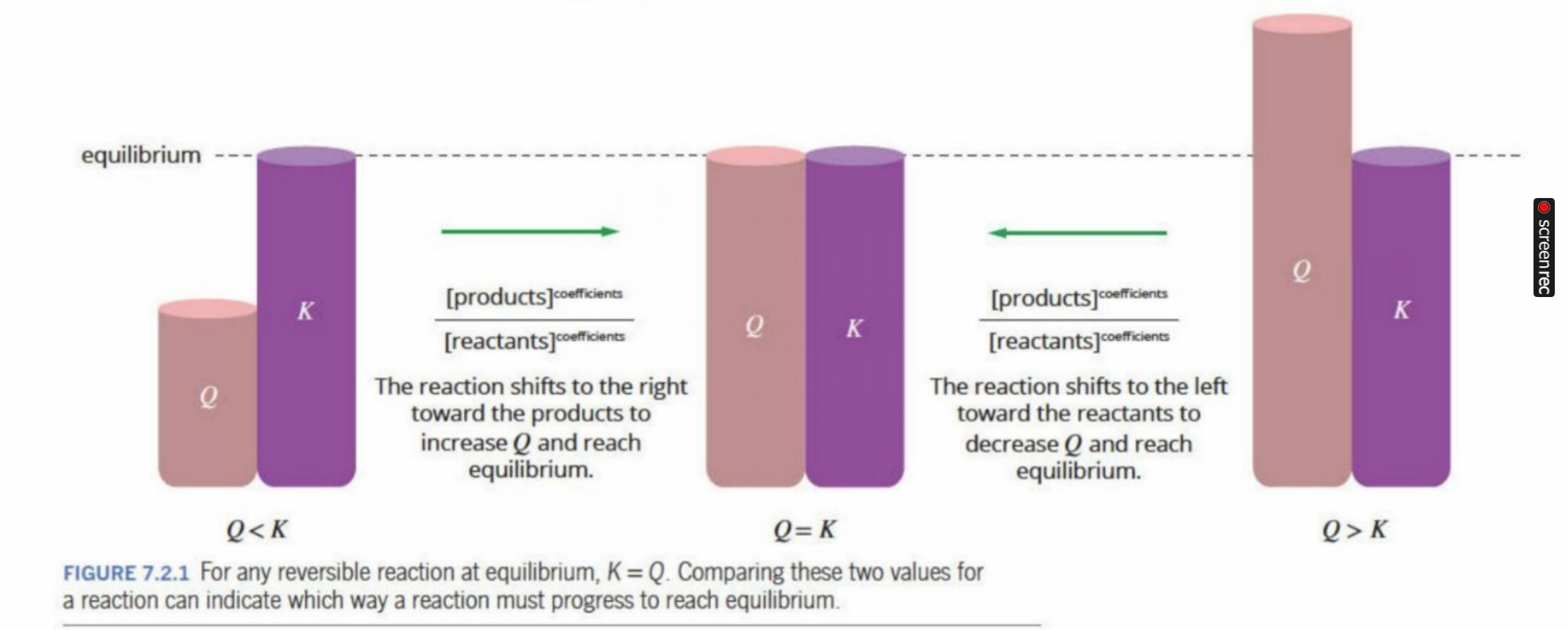
Explanation Of K vs Q
If
K>Q (right now)
that means:
The equilibrium ratio requires more products
The system currently has too few products (or too many reactants)
So the reaction shifts forward to increase the numerator (products) and decrease the denominator (reactants).
Other way: too few reactants - goes backward to reach equilibrium
Homogenous System
Where all reactants and products are in the same phase
Pure Solids and Pure Liquids In Reaction Quotient
Have constant concentrations - adding them to the equation wouldn’t result in a change - hence they are left out for simplicity
Equilibrium Yield
Amount of products present at equilibrium
Meaning Of Concentrations (and their value)
1) Signficant amount of rpoducts and reactants are made
2) Not much reaction - mostly reactants are there
3) A lot of reaction - mostly products are there
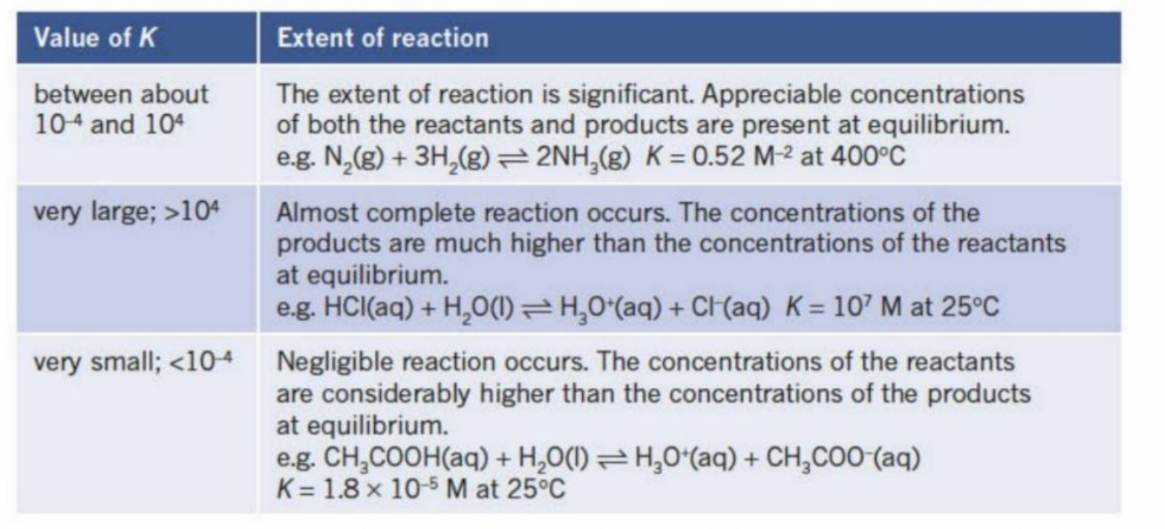
If Kc is very small
The reactants (denominator) is very large compared to the products
If Kc is very large
The products (numerator) is very large compared to the reactants
How does Temperature only affect Kc
It changes how many particles have enough energy to react (more kinetic energy)
It affects the forward and reverse reactions differently
It changes the relative rates at which forward and reverse reactions occur
When coefficients are halved
K is square rooted (from original)
When coefficients are doubled
K is squared (from original)
When doing changes to constant “K”, does that affect the units “M”?
No - they must be reworked with the equilibrium law
Example Of Using ‘Ice Box’ Method when concentrations are unknown

Le Chatlier’s Principle
If the system if offset from equilibrium by some force, it itself will adjust accordingly to attain equilibrium
Equilibrium will shift to relieve stress that it is placed under
Only works for closed systems - not when it is in contact with the open world.
Equilibrium Position
The actual amounts of products and reactants (K is simply a ration - don’t get them mixed up!)
Changes To An Equilibrium System
Adding/Removing a reactant/product
Changing pressure by changing the volume (for gases)
Dilution (for aqeuous solutions)
Changing Temperature
Adding a reactant/product
When more reactant is added, the system is temporarily not in equilibrium
Collisions between reactants occur more frequently - creates more product
But the products also collide (more particles - more collisions) to produce reactants
Equilibrium is slowly achieved (backward = forward)
Even if one is altered, it affects all concentrations
Important Note
After changes in the conditions, the system does not return to the initial equilibrium position
K value is not changed (unless temperature is changed)
All concentrations don’t go up - if reactant is added, it favors the forward reaction more (more reactants are consumed), despite the products being formed back into reactants
e.g. If one reactant is added, it favors the forward reaction, but the other reactants go down in concentration (as they were never added) - net concentration goes down
Forward Reaction Vs Backward Reaction - When product vs reactant is added
Predicting effect using equilibrium law
When more reactants are added - denominator increases - and Q is momentarily < K
This triggers a production in products (position shifts to the right)
When more products are added - numerator increases - Q is temporarily > K
More reactants are produced (Position shifts to left)
Changing Pressure by changing Volume
If pressure is higher - it favors the reaction which creates less particles, thus reducing the overall pressure
If pressure is lower - it favours the reaction which creates more particles, thus increasing the pressure
e.g. 2SO2(g) + O2 (g) - 2SO3(g)
When there is more pressure, it favours the forward reaction and as three gas particles turn into 2 - reduces pressure (equilibrium position shifts)
Applying equilibrium law to explain change of pressure
By halving the volume - all concentrations are doubled
Multiplying “2” to the power of their coefficient = to a factor of K
Pressure Changes For Liquids & Solids
They are already too tightly packed - an increase in pressure has a negligible effect on volume
When number of particles in reactants and products are equal
No matter in which direction the system shifts, the output is the same
Hence it does not oppose a volume change
When volume is doubled
The concentration of the system is halved
The system shifts to wherever more particles are being produced
Increasing pressure with an inert gas
By adding an inert gas, the overall pressure increases, but the concentration of the reactants/products is unaffected (are not squashed)
If the container is not rigid, the volume expands - lessening pressure
Dilution
When adding more water (the concentration of all gets reduced)
Position of equilibrium shifts to side where more particle are formed
To re-establish equilibrium, this is what is done
Adding temperature
The system tries to nullify the additional effect cast by this temperature
E.g. it temperature is added - it tries to “get rid” of the additional temperature by “using up the temperature” to form products (endothermic)
If temperature is taken away, it will favor the backward reaction, making heat (exothermic)
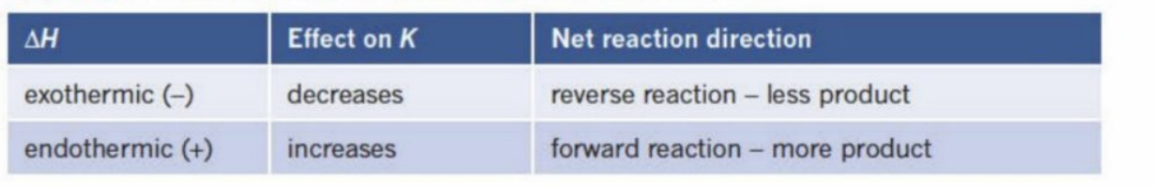
Temperature Effect On K
Catalyst Effect
Lower Activation Energy = More Successful Collisions
Favours forward and backward reactions in equal rate
Allows equilibrium to be attained faster
Does not change K, as products and reactants are formed in equal rates
When Adding Inert Gas Equilibrium Position Doesn’t Change
Though more Inert gas is added, it doesn’t affect the partial pressure of the reacting molecules
More collisions do occur but not with the reacting molecules - those are the ones that matter
Hence, it doesn’t influence the equilibrium position at all
Analogy
You have 3 blue balls and 3 red balls (the reacting molecules)
I add 10 green balls (inert gas)
I just increased the pressure within the system but I didn’t increase or influence the proportion of reacting molecules in any way
Equilibrium position remains the same