Phase 1 Drug Metabolism
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
why must we make highly lipophilic drugs water soluble
we dont want them to get too attached and stuck to fat-soluble lipid bilayer or to fatty tissues (so we add polar group/conjugation !)
how are highly water soluble drugs like antibiotics taken IV excreted?
they are excreted in the urine as they are (no addition of polar group or conjugation)
the relative contributions of direct excretion and metabolism to the drug elimination
mass-balance study
transformation of drug molecules to compounds that can be removed from the body (urine or bile)
metabolism
what influences drug metabolism (the most important determinant of drug exposure in the body)?
genetic variations
age
disease state
SEQ what happens to oral tablet immediately once you swallow?
disintegrate into powder
powder must be dissolved before being absorbed
messenteric GI tract —> portal vein
liver ( to be detoxified) —→ circulation —> tissue
what do the following stand for?
Fa
Fg
Fh
Fa= how much of the drug is dissolved
Fg = how much of the drug made it to the liver (survived GI tract)
Fh = how much of the drug made it OUT of the liver (survived liver)
what is the bioavailability if
100% of the drug dissolved
30% of the drug surviveed the GI tract
50% survived the liver
what equation did you use to solve?
F = Fa x Fg x Fh
F = 1 × 0.3 × 0.5 = 0.15
every hepatocyte has a waste channel (bile duct) which collects into bigger duct which DRAINS INTO ______________
intestine
which organ
central organ of detoxification of XEONOBIOTICS
constantly exposed to potentially harmful compounds
majority of toxins act within their cells
LIVER
a drug is converted to a more polar metabolite by
____________ by the cytochrome P450 enzymes
enzymatic __________ of the molecule uncovering polar groups in the molecule
_____________ to _______ groups such as glucuronic acid
oxidation
cleavage
conjugation polar
endogenous compound derived from the TURNOVER (making more polar/ metabolism) of heme-containing proteins such as hemoglobin, myoglobin, and ___________
Cytochrome P450
bilirubin
which phase of metabolism is called functionaization? why is it called that?
phase 1
you are adding a function to the molecule by adding a polar group
Phase 1 Reactions (functionalization):
____________ (aliphatic AND aromatic)
Modify or unmask EXISTING functional groups on the molecules
Reduction of ketones/aldehydes to ___________
Oxidation of Alcohol to ________________
Cleavage of _______ (carboxylic acid + alcohol) and ________ (carboxylic acid + amine)
Hydroxylation (can add OH to ring or chain)
alcohols
carboxylic acid
esters + amides
phase 2 reactions don’t necessarily happen after phase 1 reactions, so they can be referred to better as ____________ reactions
conjugation
what exactly are conjugation reactions?
attaching ionizable groups such as glucuronic acid, sulfates, glycine, other amino acids onto polar groups in order to inactivate the molecule
where would a parent drug be found compared to its metabolites on a reverse HPLC?
parent drug = farther right = less polar = take more time to elude out
metabolite = farther left = more polar = take LESS time to elude out
metabolites are much more water soluble as they are better prepared to be excreted in the urine. more water soluble = more polar. in reverse HPLC more polar leaves plate faster. SOO metabolites would be on the left of HPLC graph (take less time to elude out) and parent drug would be farther right (take longer to elude out)
why does attaching a sugar onto a lipophilic drug make it highly water soluble and more prepared to be excreted int he urine or as bile?
sugar has ALOT of OH groups
within hepatocytes of the liver, which organelle do most of the enzymes involved in phase 1 and phase 2 metabolism come from?
why does this make sense?
most metabolizing enzymes come from the endoplasmic reticulum
this makes sense because ER carries the most fat, so the drugs will accumulate in the ER where they are metabolized
is the fractional contribution to drug metabolism directly related to the amount of protein expressed?
for example, if there is few 2D6 does that mean 2D6 is only responsible for a very small portion of drug metabolism?
NO
2D6 takes up a very small portion of the total amount of metabolizing agents but it is responsible for a significant portion of metabolism that takes place
which ER enzyme is responsible for MOST lipophilic drug metabolism AND makes up most of the enzymes?
Cytochrome 3A4
where does cytochrome P450 get its name?
maximum absorption of pigment of the reduced enzyme when bound to carbon monoxide
makes sense bc/ if cytochrome P450 is responsible for oxidation then it itself is REDUCED
what enzyme MUST CYP450 3A4 react with (redox partner) in order to preform its catalytic function?
what does this redox partner do?
CYP450 reductase
donates two electrons FROM NADPH to CYP450 using cpenzymes FAD and FMN
CYP450 reductase donates 2 electrons to CYP450 in order to activate it.
How does it donate 2 electrons?
CYP450 reductase is a flavoprotein
it takes 2 electrons from NADPH and gives it to FAD and then to FMN which donates them to CYP450 one at a time
if we didn’t have CYP450 as an intermediary then CYP450 would receive both electrons from NADPH at once, which would NOT activate CYP450 MUST CONSEQUTIVELY receive electrons
essential cofactor that is generated from the cellular metabolism of nutrients such as glucose and fatty acids
_______ is required to drive CYP450 and FMO-monooxygenase activities
Nicotinamide Adenine Dinucleotide Phosphate (NADPH)
what makes up Flavin Adenine Dinucleotide (FAD)
how do these two molecules come together?
FMN (flavin mononucleotide) + AMP (adenosine monophosphate)
riboflavin is phosphorylated then attaches to AMP
is FAD a prosthetic group or a stoichiometric group?
what is an enzyme reffered to if it has FAD (FMN + AMP) attached to its active site?
prosthetic (stays attached to enzyme)
flavoprotein
Cytochrome P450 is activated once it receives 2 electrons one at a time.
How does it receive these electrons?
What prevents Cytochrome P450 from receiving both electrons at the same
Cytochrome P450 Reductase takes 2 electrons from NADPH and donates them to CYP450
it is able to donate one at a time by shuffling the electron from its prosthetic groups going from NADPH then FAD then FMN and FINALLY then to CYP450
NADPH —> _______—→ _____/______—→ CYP450
FADH2 —> FADH⋅/FMN —> CYP450
FADH⋅/ FMNH⋅ is also called FADH2 which is also called
semiquinone
how does CYP3A4 act as a “vacuum cleaner” for the membrane?
where is the CYP450 active site located?
lipophilic drugs will stick to hydrophobic lipid tails of membrane and CYP450 will polarize these drugs taking them out of the membrane—> back into circulation—> excreted as urine
CYP450 active site located WITHIN membrane
the oxygen that is inserted into drugs following oxidation cytochrome P450 comes from
molecular oxygen (split oxygen)
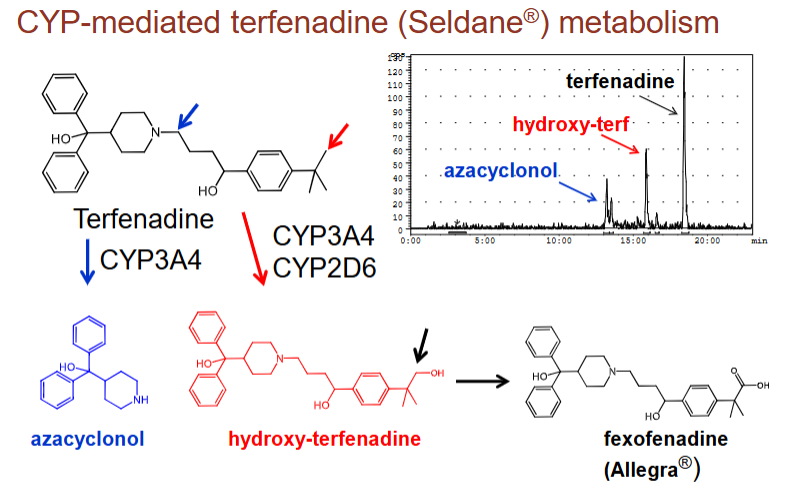
what are two ways CYP3A4 breaks down terfenadine (antihistamine that killed patients):
add a functional group through addition of molecular oxygen (hydroxy-terfenadine)
split the molecule to uncover functional groups (azacyclonol)
where is the active site of CYP450 located?
where is the active site of UGT (phase 2 metabolism) located?
embedded within the membrane (lipophilic tail)
on the membrane (has been polarized by CYP450 but is still within the mwmemembrane)
________ ______ and _______ of drugs or metabolites is a main determinant of in vivo disposition
membrane permeability and lipophilicity
what determines whether or not a phase 1 metabolite needs to be further polarized through conjugation?
membrane permeability
if a drug still cannot leave the membrane upon functionalization (addition of polar group) than it may be further polarized through conjugation
can compounds be eliminated directly by UGT transferases or must they first undergo phase 1 reaction first?
YES they can
isn’t ALWAYS phase 1 —> phase 2
Drug Metabolism: Specificity vs Efficiency
low/high substrate specificity, capable of accepting large varieties of structurally diverse substrates
low/high chemo-specificity and/or product specificities (capable of catalyzing the formation of more than one type of _________ ___________)
low catalytic rates can be compensated for by having extremely high ________ levels in tissues of ________
preference for ___________ substrates
expression can be induced by multiple _________; inducers can themselves be substrates (_______)
low
low functional group
expression disposition
lipophilic
xenobiotics autoinduction
what are the five fates of a drug undergoing metabolism?
lidocaine, procaine, phenytoin, phenobarbital, midazolam
codeine, acetaminophen (NAPQI), cyclophosphamide
enalapril, sacubitril, phenacetin, levodopa, tamoxifen, efavirenz
tefenadine, loratadine, digitoxin, amitryptaline, diazepam
ipronizaid —> isonizid
active to inactive or less active
active to more active
inactive (prodrug)—> active
active —> active on the same target but with different pharmacologic activity
active —> active on DIFFERENT target (antidepressive —> anti-infective)
which two enzymes can be found within the cytoplasm of hepatocytes?
sulfotransferases and glutathione transferases
which enzyme is found within the lysosomes of hepatocytes?
peptidase esterase
which enzyme is found within the mitochondria of the hepatocyte?
amino acid conjugation
which enzymes are found within the endoplasmic reticulum of the hepatocyte?
cytochrome P450
glucaronosyl transferase
glutathione trasnferase
is cytochrome p450 only found in the lipophilic endoplasmic reticulum?
NO it is also found within the mitochondria (cholesterol and steroidogenesis within mitochondria)
what is induction and what is repression in terms of CYP450?
induction = increased expression of CYP450
repression = decreased expression of CYP450
the ER can proliferate (increase total protein and lipid) under conditions of _______________
explain what this means
induction
CYP450 majority located in the ER can increase in levels with treatment which INDUCES metabolism (which is why an increased dose = increased clearance)
what happens to levels of CYP450 in patients experiencing inflamation?
decreased CYP450 causes patients to be more sensitive to treatment, longer exposure to drug because drug is not removed by CYP450
A patient is taking drug A and drug B
in order to determine whether or not the drugs can be taken at the same time it is crucial to know which enzymes the drugs are metabolized by. Why?
How can you figure out which enzyme is responsible for the metabolism of drug A and B?
if drug B inhibits the enzyme that drug A is metabolized by then drug A will reach toxic levels
incubate compound with hepatocyte and observe METABOLITES (if the drug metabolite is glucoronidated then you know it underwent conjugation with UGTs if hydroxylated CYP450)
there are MANY CYP450s how are they named?
ex. 3A4 (where does each letter come from?)
named based on AMINO ACID SEQUENCE
3= family (share more than 40% sequence with other CYP450s)
A = subfamily (shares more than 55% sequence identity)
4= individual P450 — order in which it was cloned
STRUCTURE OF CYP450
reside in the ER but their active site is found in the _________ (what keeps it there)
what binds to their active site?
what are its two domains?
membrane (long tail N-terminal anchor keeps it attached to the membrane)
drug and oxygen (cofactor)
FAD and FMN domains (will transfer 2e- from NADPH one by one)
once CYP450 is activated from acquiring 2 electrons from NADH using CYP450 reductase what reaction takes place?
NADPH + H+ + O2 + RH —→ H2O + ROH + NADP+
Oxygen splits creating alcohol and water
why can’t CYP450 accept both electrons at once?
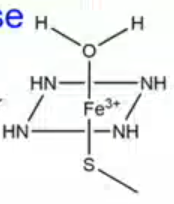
what type of interactions keep CYP450 together?
Fe3+ is posively charged and is balanced by 6 negative charges
4 negative charges come from the Nitrogens that form the perforin ring
1 negative charge comes from the sulfhydryl group on the cysteine residue
1 negative charge comes from the water at the top
ELECTROSTSTATIC INTERACTIONS KEEP CYP450 TOGETHER IN INACTIVE STATE
SEQ CYP450 catalytic cycle (hydroxylation)
substrate binding
1st e- transfer from NADPH using CYP450 reductase (reduces _____ ——> ______)
CYP450 is now able to bind to _______ and form a ______ bond
Iron then donates an electron to ______ breaking apart the double bond leaving ____
CYP450 reductase donates THE SECOND proton to _______ from NAPH making it _______
two protons interact with the enzyme substrate oxygen complex —> 2 electrons and an oxygen from the complex get transferred onto the protons creating a ______ molecules
the remaining oxygen is transferred over to the substrate (RH) —> RELEASE SUBSTRATE (_____) !
Fe3+ —> Fe2+
O2 coordinate covalent
O2-
O2 —> O2-
water
ROH
SEQ CYP450 CATALYTIC (highlighted steps)
substrate binding
first _____ _______ (_______ reaction)
_____ binding
_________ intermediate
_________ intermediate (EXTREMELY REACTIVE) Fe4+
RADICAL REBOUND transfer of Fe-ligated ______ atom to the substrate (____)
product release ! (_____)
electron transfer reduction
O2
hydroperoxyl
oxyferryl
oxygen RH
ROH
Reactions Catalyzed by CYP Enzymes
carbon oxidations
oxidation of sp3 hybridized carbon atoms
C-sp3 _________
C-sp3 ________
______ of benzylic position
oxidation of sp2 hybridized carbon atoms (____________)
_________ of sp hybridized carbon atoms (acetylenic bonds)
hydroxylation
desaturation
oxidation
epoxidation
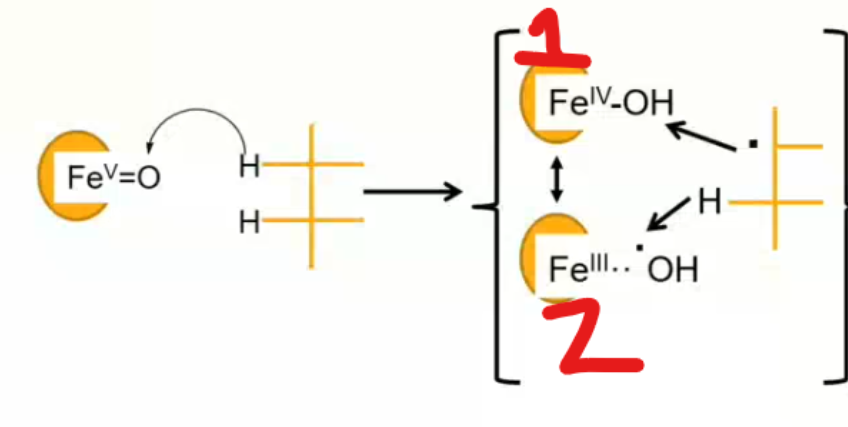
between 1 and 2 which scenario leads to hydroxylation of the substrate? which leads to the formation of an unsaturated double bond?
reaction 1 leads to hydroxylation
reaction 2 leads to unsaturation
if CYP450 is hydroxylating a benzene with a withdrawing group, where would the OH be in relation to the withdrawing group (deactivating)
ex. —Cl, Br, F or =O or cyano, nitril, sulfonyl, CF3, NR3
para or ortho (right next to)
if CYP450 is hydroxylating a benzene with a donating group, where would the OH be in relation to the withdrawing group (activating)
ex. amine, ammide, —O (alkoxide) , ester, methyl, hydroxy
META (skip one)
rearrangement where a hydrogen atom on an aromatic ring undergoes an intramolecular migration
NIH shift (aromatic hydroxylation)
what are some additional reactions that are catalyzed by CYP enzymes :
Nitrogen ______ and oxidative _________
oxygen ____________
oxidative ____________, _________, _____________, _____________
reductive reaction
nitro- , _____- reduction
reductive de__________
nitrogen de-alkylation and oxidative deamination
oxygen dealkylation
oxidative deSULFINATION, deHALOGENATION , deNITRIFICATION
azo
dehalogenation
explain what happens during N-dealkylation reaction by CYP450 enzymes
CYP450 hydroxylates molecule (ex. terfenadine) next to amine
amine and OH are reactive next to each other SO
HYDROLYSIS REACTION SPLITS THEM INTO
SECONDARY AMINE + ALDEHYDE
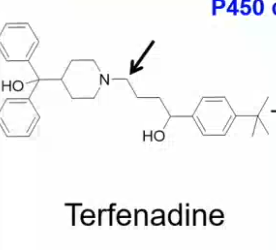
which metabolic reaction catalyzed by CYP450 will take place?
which two metabolites will come out of this reaction?
N-dealkylation
SECONDARY AMINE and ALDEHYDE
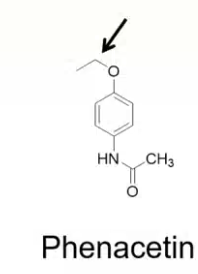
which metabolic reaction catalyzed by CYP450 will take place?
which two metabolites will come out of this reaction?
O- dealkylation
ALCOHOL + ALDEHYDE
what allows CYP450 to carry out reactions that other metabolic enzymes cannot?
HIGHLY REACTIVE OXYFERROL (Fe4+) intermediate
what are the reactants and products of metabolism carried out by Flavin Containing Monoexygenases (FMO)?
X+ O2 + NADPH/H+ —> XO+ H2O + NADP+
X= N, S, P, Se
although FMO cannot hydroxylate molecules through the cleavage of C-H bonds, it is able to directly hydroxylate which 4 molecules?
the hydroxylated molecule is not the end product, what is?
N,S,P,Se
end result of FMO metabolism = oxides (NO, SO, PO, SeO), water, and NADP+
P450 has ______ group in active site
FMO has _______ in active site
heme
FAD
can FMO get its electrons DIRECTLY from NADPH or must it use an intermediate enzyme similar to CYP450 reductase to receive the electrons?
where does NADPH bind to on FMO?
can get electrons DIRECTLY
binds near carboxylic acid end in glycine rich area
the FAD (flavin adenine dinucleotide) which sits in the active site of FMO (flavin containing monooxygenases) is made up of which two molecules?
flavin mononucleotide (FMN) and adenosine monophosphate (AMP)
AMP attaches once riboflavin is phosphorylated
FMN + AMP —> FADH2 oxidized will give you FAD
SEQ: FMO Reaction Cycle
______ binds and reduces ______ the oxidized ______ remains bound to the active site during the cycle to stabilize the ___-_____ complex
the ________ intermediates is capable of inserting an oxygen atom into heteroatoms (N,S,P,Se) but NOT capable of C-H bond cleavage
RATE LIMIITNG STEP: ______ and _______ is removed from enzyme
NADPH FAD NADP+ FAD-OOH
hydroperoxyy
NADP+ and water
what reaction does Flavin-Contianing MonoOxygenase (FMO) catalyze?
how many FMO enzymes are there?
can CYP450 create the same metabolites that FMO can (N- oxidase)?
the oxygenation of “soft” nucleophilic heteroatoms (N, S, P, Se)
5 (FMO 1-5)
YES
if a metabolite has a hydroxylated carbon which enzyme was used to catalyze the reaction?
CYP450 NOT FMO (FMO can only hydroxylase N, S, P, Se)
Monoamine Oxidases (MAO):
Are MAOs also located in the ER like CYP450 and FMOs?
How many MOAs exist?'
Which cofactor is COVALENTLY BOUND through sulfhydryl on cysteine residue to the active site of MAO? is it prosthetic?
NO MAOs are located in the mitochondria attached to outer membrane by tail
2 (A and B)
FAD YES
what catalytic reaction are MAOs responsible for?
catalyze oxidation of endogenous neurotransmitters and exogenous amines (CNS acting drugs)
catalyzes the oxidative DEAMINATION of neurotransmitters (deactivation) and nitrogen-containing drug molecules in the ABSENSE of NADPH
monoamine oxidase (MAO)
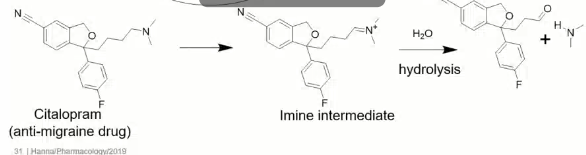
If NADPH is not used in metabolic reactions catalyzed by MAO where do the electrons come from to begin the reaction?
Amines donate their electrons to the molecule converting themselves into positively charged imines
MAO Catalysis SEQ:
No NADPH so amine donates electrons to the molecule converting itself into _____________ intermediate
HYDROLYSIS reaction creates __________ and _________
the oxidation of the substrate is coupled with the reduction of the covalently bound ______ prosthetic group
imine
aldehyde and amine
FAD
Molybdenum Hydroxylases: Aldehyde Oxidase (AO) + Xanthine Oxidase (XO)
Where are they located within liver cells?
They are responsible for the ___ or ____ CARBON oxidation of ________ (R1R2C=NR3)
cytosol
alpha or gamma imine
if you see a metabolite that looks like an amide within a ring which enzyme was used for its metabolism?
xanthine oxidase
_____ and ______ play a major role in the metabolism of heterocyclic amines (very common substituents in drug molecules)
aldehyde oxidase (AO) and xanthine oxidase (XO)
Both animals and humans have the metabolic enzymes Aldehyde Oxidase (AO) and Xanithine Oxidase (XO).
Can you administer a drug to an animal and extropolate the metabolism data of animal AO and XO from preclinical species to humans?
Can animals response be used to predict human response of XO and AO?
NO because they are vastly different from species to species
an animal’s XO may not be as effective as a humans XO
_________ turns caffeine into ___________ which is turned into ______ by XANTHINE OXIDASE
CYP1A2
1-methylxantihine
1-methyluric acid
SEQ Guanosine Metabolism using Xanitihine Oxidase a
guanosine —→ hypoxanthine——> xanthine (using XO) ——> uric acid (using XO)
what is the difference between xanthine dehydrogase (XDH) and Xanithine oxidase?
they are the SAME enzyme converting xanthine to uric acid but
fXO—> oxygen accepts electrons becoming H2O2
XDH—> NAD+ accepts electrons becoming NADH
what do dehydrogenase enzymes do?
oxidation reactions
result in the formation of reduced species such as NADPH or NADH
what three things do all xanthine oxidase, aldehdye oxidase, xanthine dehydrogenase, and aldehyde dehydrogenase have?
FAD
1 atom of Molybdenym (MoVI (=s =o 2+ and OHH)
4 non heme Fe = 2 Fe/S2 clusters
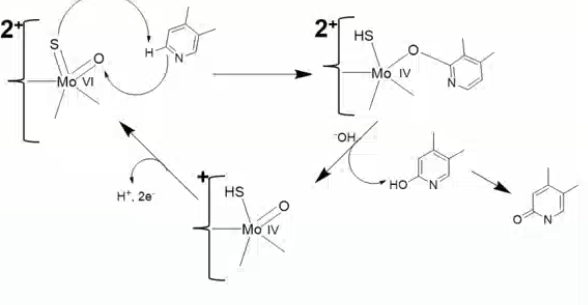
which enzyme catalyzes the following reaction?
xanthine oxidase
MICROSOMAL P450s
Accessory Proteins:
Prosthetic Group:
Cellular Localization (where is it found?) : '
Reducing cofactor:
Source of Oxygen:
Accesory Protein: NADPH- CYP450 reductase and Cytochrome b5 (can also donate 2nd electron)
Prosthetic Group: heme
Cellular Localization: endoplasmic reticulum
Reducing cofactor: NADPH
Source of Oxygen: O2
which metabolic enzymes use O2 as their source of Oxygen?
which use H2O?
O2 = CYP450 and FMO
H2O= MAO and XO/AO/XDH
FMO
Accessory Proteins:
Prosthetic Group:
Cellular Localization (where is it found?) : '
Reducing cofactor:
Source of Oxygen:
none
FAD (FMN + AMP)
endoplasmic reticulum
NADPH
O2
MOA:
Accessory Proteins:
Prosthetic Group:
Cellular Localization (where is it found?) : '
Reducing cofactor:
Source of Oxygen:
none
FAD
Mitochondria
none (make imine by amine donating electrons)
H2O
AO/XO/XDH:
Accessory Proteins:
Prosthetic Group:
Cellular Localization (where is it found?) : '
Reducing cofactor:
Source of Oxygen:
none
FAD, 1 molybdenum atom, 4 Fe (not heme) as 2 Fe2S2 cluster
cytosol
none
H2O
when enzyme has heme as its prosthetic group?
CYP450
which enzyme (S) have FAD as their prosthetic groups?
FMO and MAO
where are each of the enzymes following found within the liver?
Monoamine Oxidase (MOA)
Aldehyde Dehydrogenase (ADH)
Xannthine Oxidase (XO)
Flavin containing MonoOxidase (FMO)
Xanthine Dehydrogenase (XDH)
CYP450
Aldehyde Oxidase (AO)
mitochondria
cytosol
cytosol
endoplasmic reticulum
cytosol
endoplasmic reticulum
cytosol
Reducing equivalents are derived from the ________ of substrates, electron acceptors are either ___ or _____ (XDH) to return the enzyme to the oxidized form (resting state)
oxidation
O2 or NAD+