Fischer Esterification Theory
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Fischer esterification
a reaction that converts a carboxylic acid into an ester.
what occurs during esterification
the -OH portion of the carboxylic acid is replaced by an -OR group from an alcohol.
what is the byproduct of esterification and what does it do?
The byproduct of this reaction is water
since water is also a nucleophile, it can add back into the compound and hydrolyze the newly formed ester to regenerate the starting carboxylic acid
how to bypass water reforming the carboxylic acid after esterification?
run the reaction in excess alcohol to drive the reaction towards product
goal of the experiment
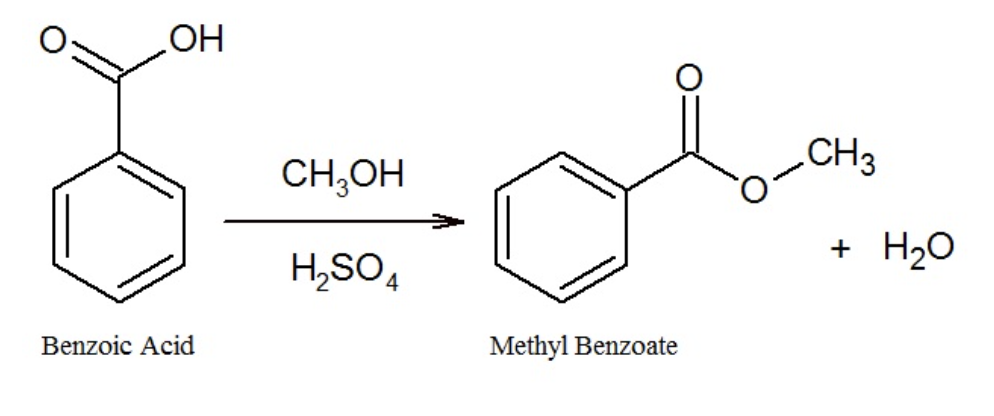
synthesis of methyl benzoate from benzoic acid and methanol (esterification)
product will be isolated and analyzed with IR & gas chromatography
experiment reaction
heating block instructions
There are multiple indentions on the heating block, make sure you use the one that fits the round bottom flask properly.
water and the condenser
Ensure that you use the metal clips to secure the water hoses on the condenser
Water goes in at the lower end and out at the higher end
why is only a slow flow of water necessary?
If the water pressure gets too high the hoses will come off and flood the hood.
what should you do after handling sulfuric acid?
CHANGE YOU GLOVES IMMEDIATELY AFTER HANDLING SULFURIC ACID!