C2 identifying the ions in an ionix compound using flame tests
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Ionic compounds are made of what
Cation and anion
Cation have characteristic colour when held in flame
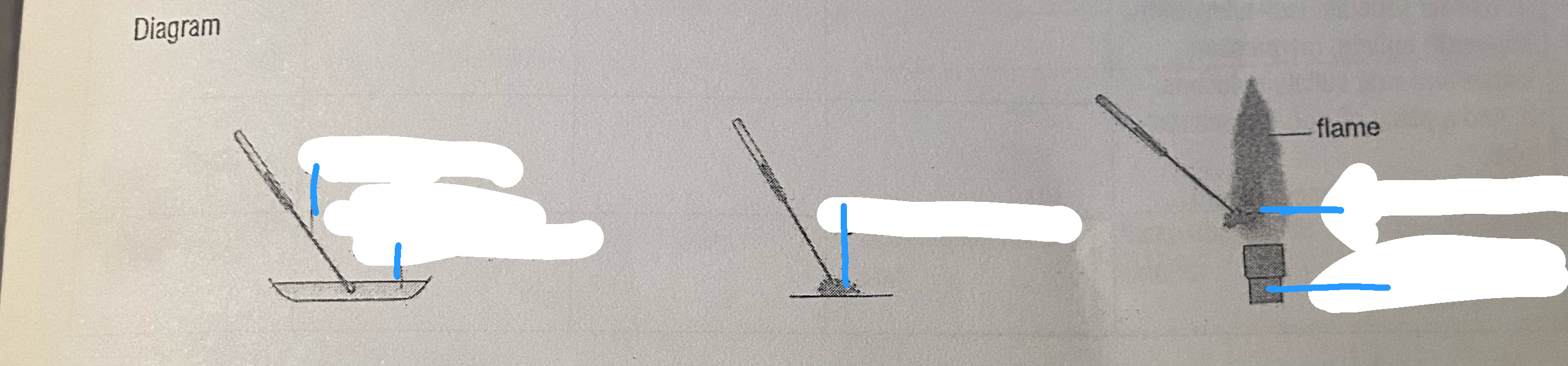
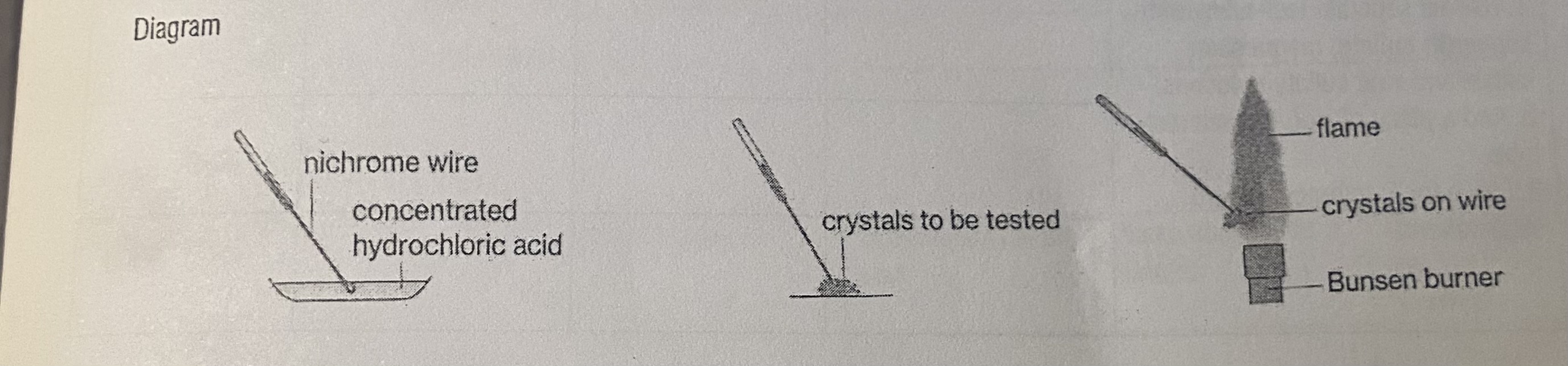
Method
Take a piece of nichrome wire with a loop at one end.
Dip the loop in the concentrated hydrochloric acid and place in the hot blue flame of the Bunsen burner.
Repeat until the flame is no longer coloured, i.e. impurities are removed.
Dip the clean nichrome wire in concentrated hydrochloric acid and the wire onto the solid ionic compound which is sitting in a watch glass.
Hold the loop with the solid ionic compound in the hottest part of the flame and record the characteristic colour.
Repeat for all the solid ionic compounds available, cleaning the wire loop each time.
Sodium observation
Yellow/orange
Calcium observation
Brick red
Potassium observation
Lilac
Copper observetion
Blue-green
Lithium observation
Crimson