Week 1B: Water, Acids, Bases and Buffers
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
How much of the human body weight is from water?
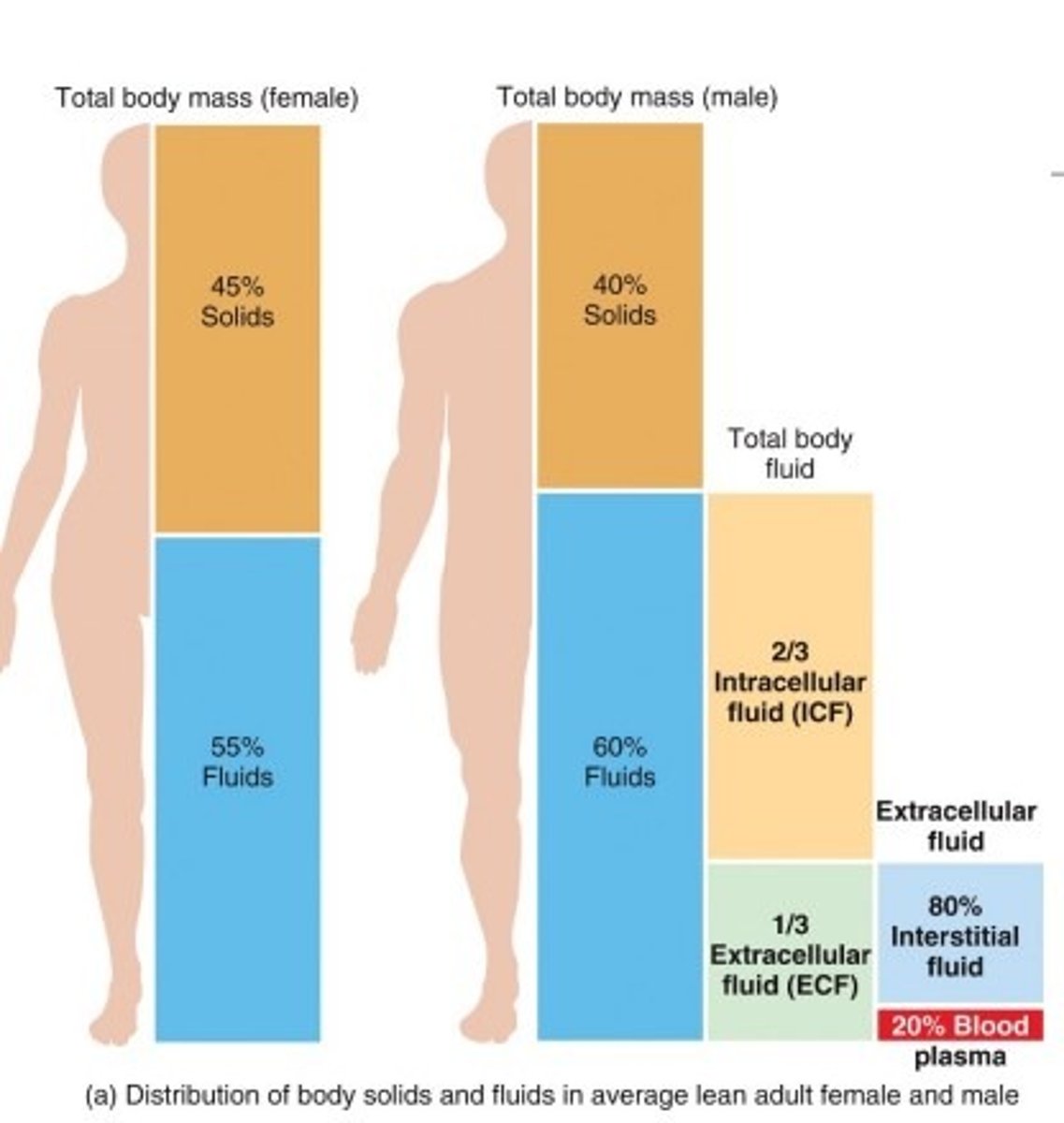
Approximately 60%
How is water distributed in the human body?
Water is divided into two compartments in the human body
Intracellular compartment (inside of cells) ~25L
Extracellular compartment (outside of cells)
-Interstitial fluid (fluid between cells) ~10L
-Fluid in Blood ~5L
What is the function of water being compartmentalized in the human body?
Compartmentalization helps control the concentration of molecules in different areas, increasing the probability that certain reactions will occur to maintain homeostasis
How does water act as a solvent?
Electrons in H-O bonds form weak polar covalent bonds as electrons are more attracted to the oxygen molecule due to greater electronegativity.
These polar covalent bonds can be broken and reformed allowing water to dissolve other polar molecules in water by forming a hydration shell, whereas the oxygen (negative dipole) or hydrogen (positive dipole) of the water faces the ions it surrounds
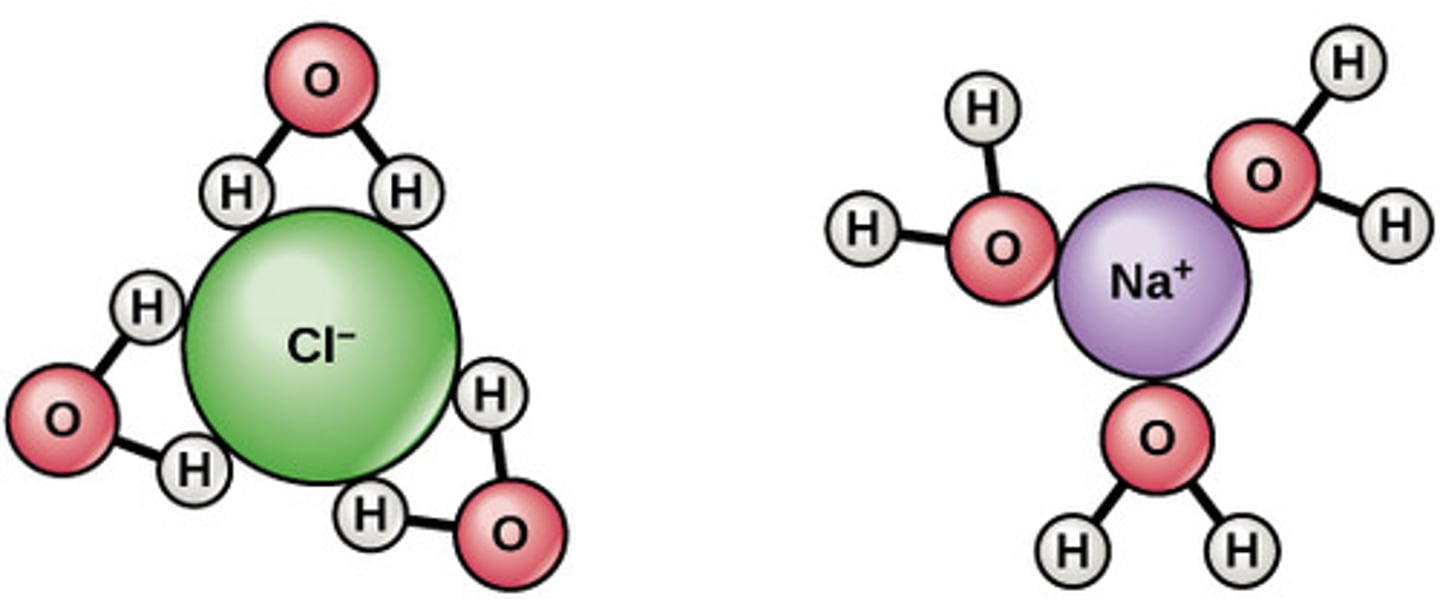
What are electrolytes?
Anions and cations that water dissolves
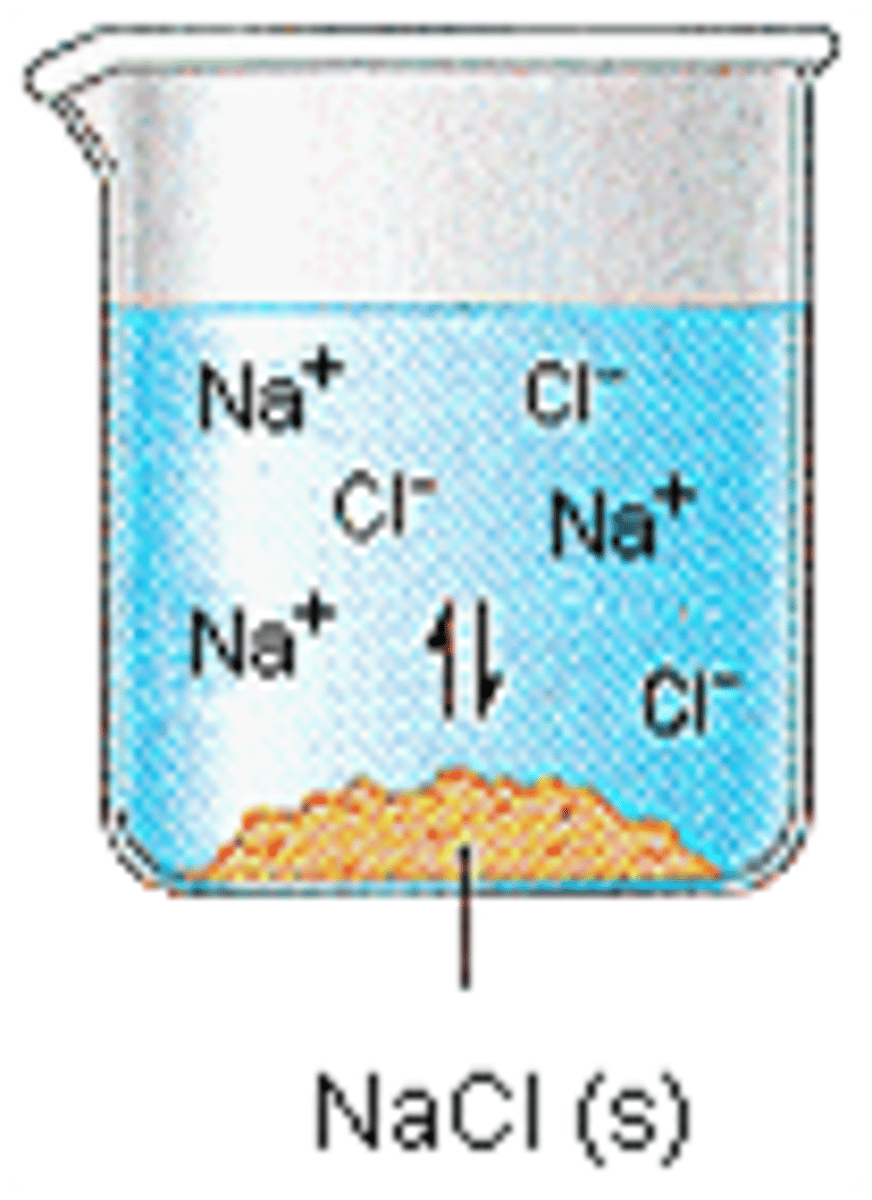
What is osmolarity?
Concentration of all dissolved solutes in the blood (electrolytes, proteins), water maintains osmolarity in different compartments via osmosis
What is osmosis?
A process where water moves to the higher concentration of solutes (osmolarity) to maintain equilibrium between two solutions
What is Kd? How is it calculated?
Dissociation constant
Measure of how easily a molecule breaks apart on its own
(Conc/Mol dissociated #1)(Conc/Mol dissociated #2) / (Mols leftover)
Ex: 20 molecules of H20 start with , dissociated into H + OH with 10 mols leftover
(10)(10)/(20-10) = 10
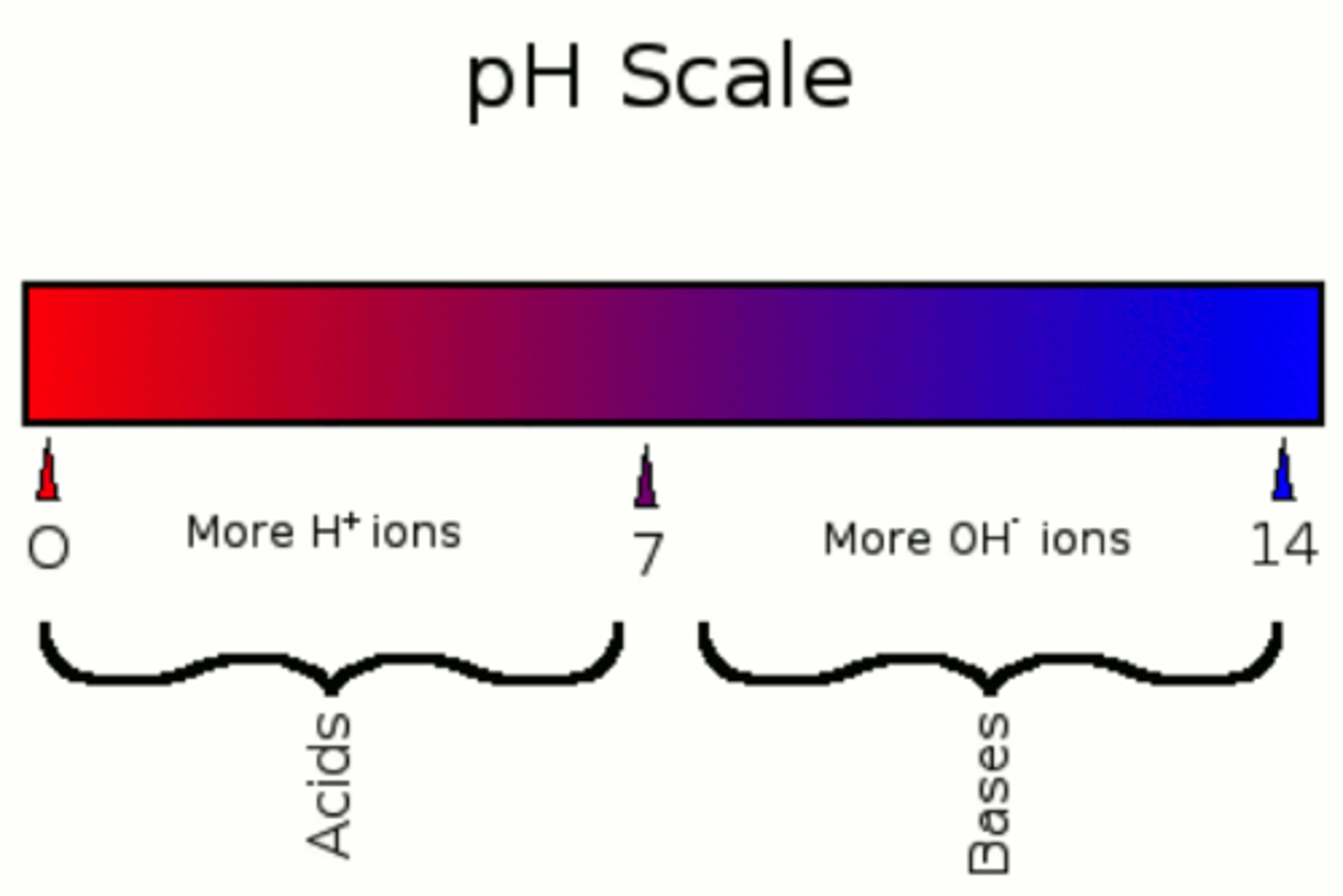
What is pH of a solution? How is it calculated?
Measure of acidity or alkalinity of a solution/ how many hydrogen ions are in the solution
pH= -log[H+] (pH is -log of hydrogen ion concentration)
What is the normal pH range for blood? Why is this so?
Blood pH = 7.35-7.45
Blood has many electrolytes, acids, and other molecules dissolved in it, therefore, more basic than water
What are acids vs bases?
Acids - Molecules which can release an H+ ion into a solution
Bases - Molecules which can accept an H+ ion from a solution
What are strong vs weak acids?
Strong acids - strong electronegativity, can pull electrons completely away from H+
Weak acids - less electronegative, less likely to completely dissociate, involved with buffers
What is Ka? How is it calculated?
Equilibrium constant
Tendency for weak acids to dissociated into a conjugate base and a hydrogen ion
(Conc/Mol dissociated #1)(Conc/Mol dissociated #2) / (Mols leftover)
Ex: 20 molecules of HA start with , dissociated into H + A with 10 mols leftover
(10)(10)/(20-10) = 10
When is a buffer the most effective according to the Henderson-Hasselbalch equation?
Buffers are most effective when 1+-pH=pKa, which occurs when 50% of the acid is dissociated, resulting equal amounts of the acid and it's conjugate base
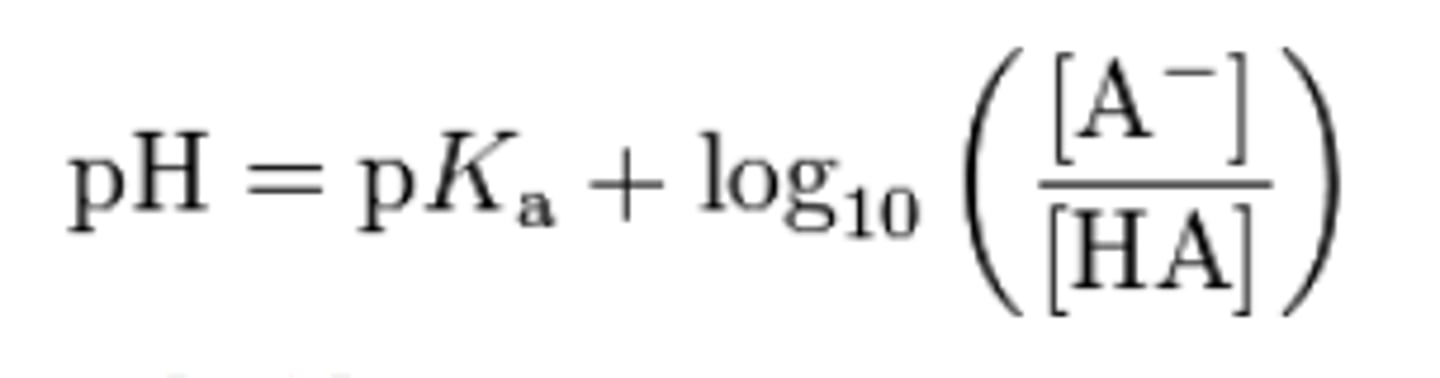
What are buffers and their role?
Buffers are a combination of weak acids and their conjugate base
Buffers resist changes in pH, and are most effective when 1+-pH=pKa as equal amounts of acid and base (50% dissociated) are present, meaning that protons can be accepted and donated to maintain pH when H+/OH- ions are introduced into the solution
It is critical to our how body maintains pH
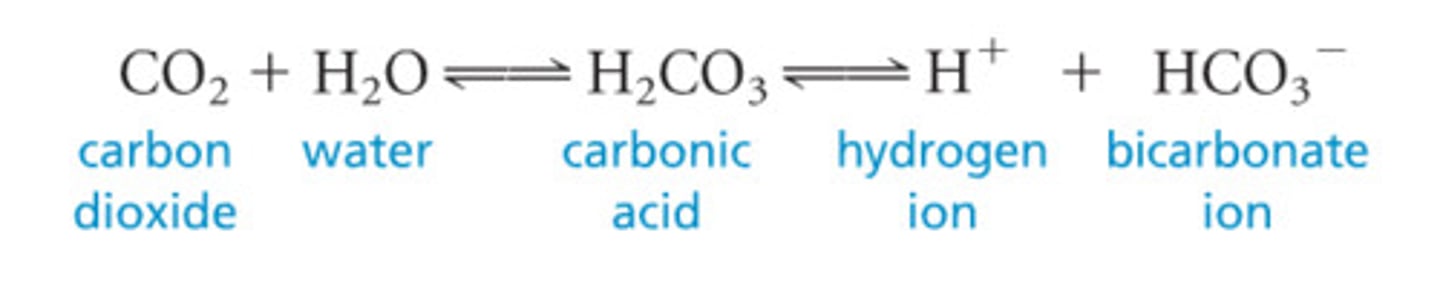
What is the chemical equation, pKA, and role of the bicarbonate buffering system?
CO2 + H20 (more basic) <- carbonic anhydrase-> H2CO3 <-> HCO3- + H+ (more acidic) pKa = 7.4
Major buffer system for maintaining pH of blood (7.35-7.45)
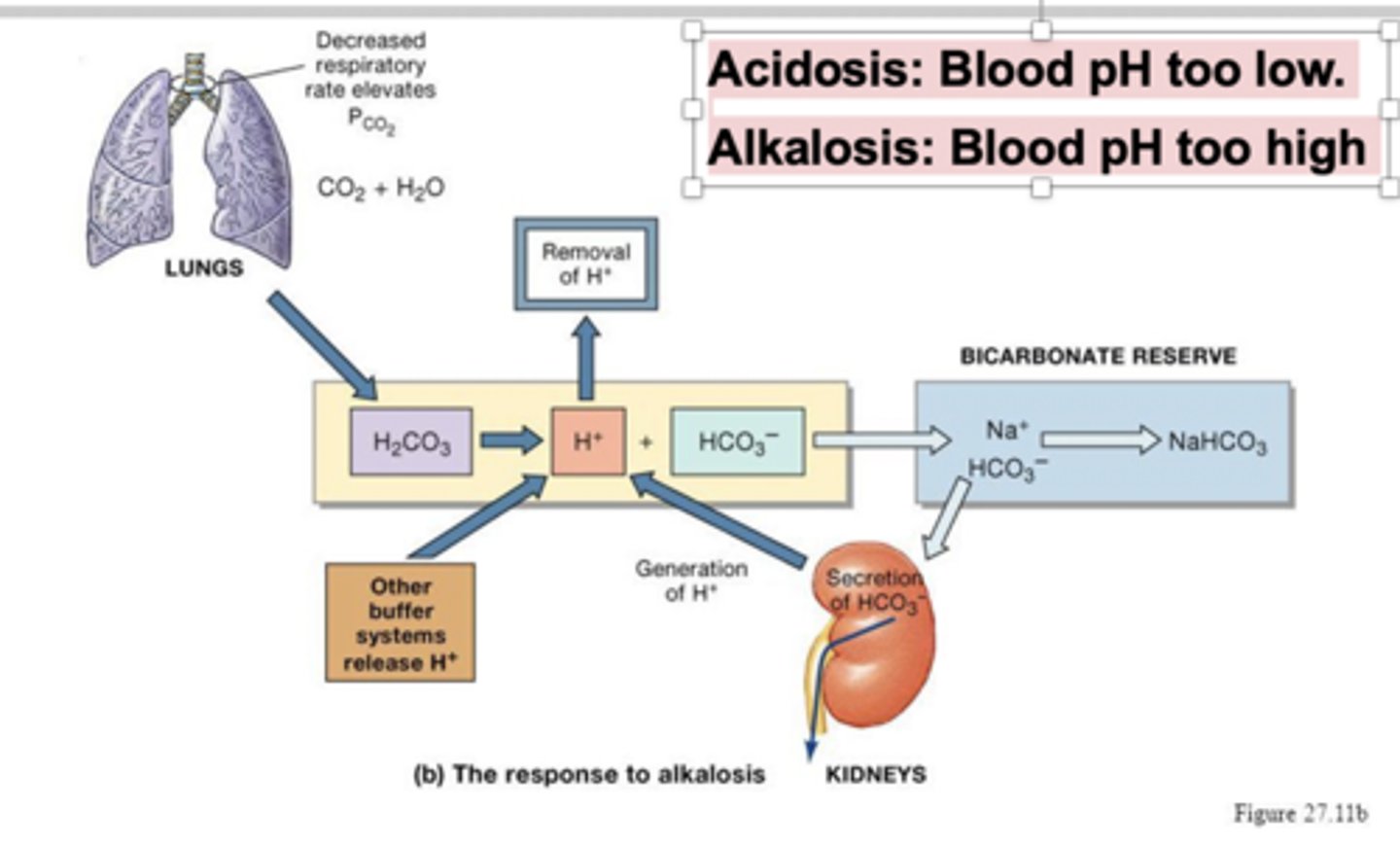
How does the bicarbonate buffering system react when blood is under acidosis?
HCO3- + H+ -> H2CO3 -> CO2 (released via lungs) + H20
HCO3- + H+ can be released via urine during acidosis
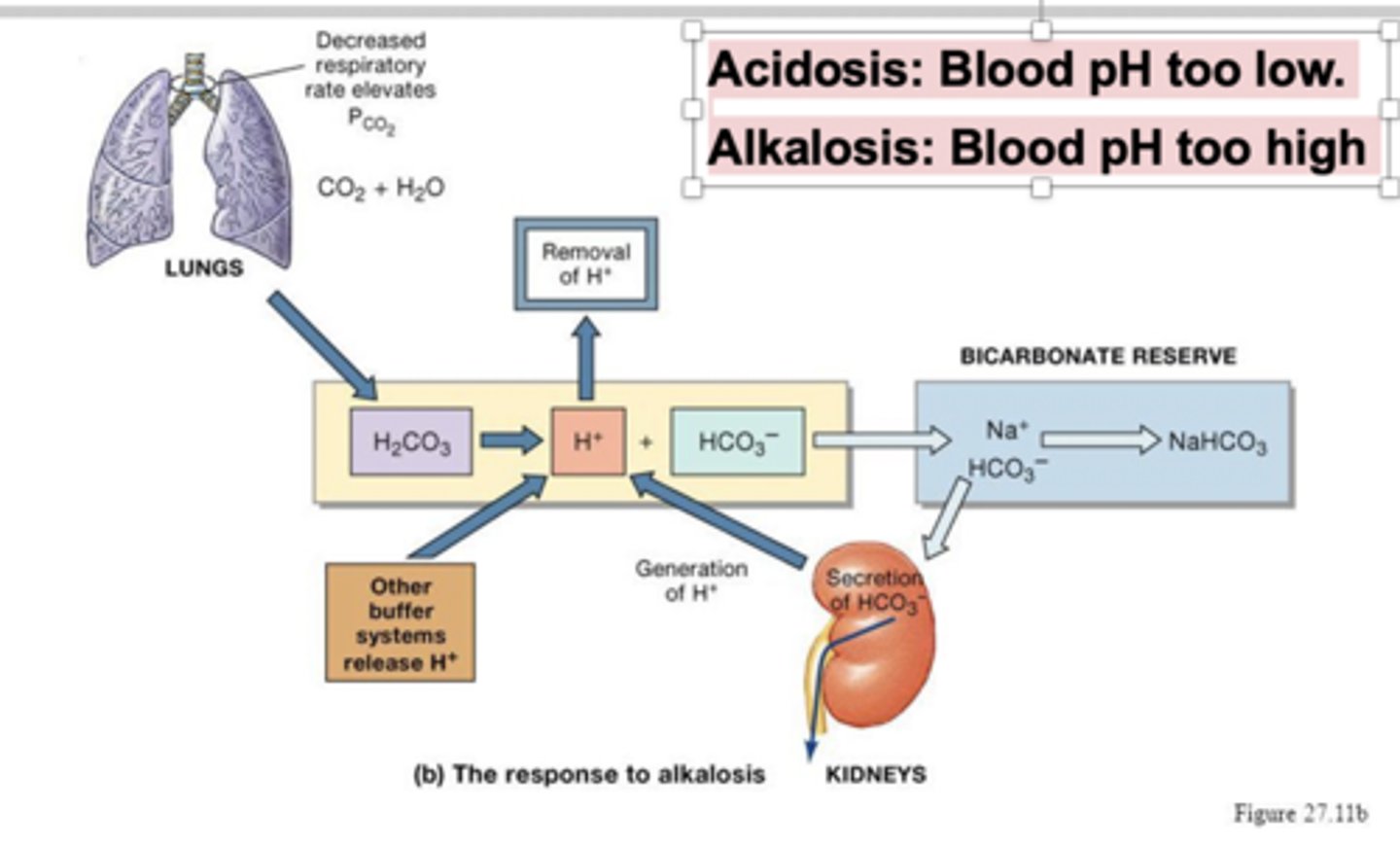
How does the bicarbonate buffering system react when blood is under alkalosis?
CO2 + H20 -> H2CO3 -> HCO3- + H+
What enzyme is responsible for converting CO2 + H2O into carbonic acid in the bicarbonate buffer system? Where is this enzyme produced?
Carbonic anhydrase, produced by rbcs and kidney cells
What are three examples of buffer systems in the human body?
Dihydrogen phosphate-hydrogen phosphate system - active inside cells
(H2PO4- <-> HPO4 2- + H+ pKa = 6.8)
Proteins -> amino side chain that accepts H+, lowering acidity
Bicarbonate buffering system - regulates pH in blood
(CO2 + H2O <-> H2CO3 <-> HCO2+ H+ pKa = 7.4)
What can cause metabolic acidosis?
Excessive production of ketoacids or lactic acid, or loss of bicarbonate (excessive diarrhea)
What can cause metabolic alkalosis?
Ingesting basic compounds (Bleach), or retaining bicarbonate, or excessive vomiting
What can cause respiratory acidosis?
Conditions where CO2 is retained (pt is unable to exhale it in pneumonia or emphysema)
What can cause respiratory akalosis?
Hyperventilation due to stress, drug overdoses, and fever (increased metabolic demands)
How is metabolic acidosis compensated?
Respiratory Compensation
Respiratory Co2 elimination (hyperventilation)
How is metabolic alkalosis compensated?
Respiratory Compensation
Respiratory Co2 retention (hypoventilation)
How is respiratory acidosis compensated?
Metabolic Compensation
Renal bicarbonate retention and hydrogen elimination
How is respiratory alkalosis compensated?
Metabolic Compensation
Renal bicarbonate elimination and hydrogen retention
Which organ is responsible for respiratory compensation in the bicarbonate buffer?
Lungs
Which organ is responsible for metabolic compensation in the bicarbonate buffer?
Kidney
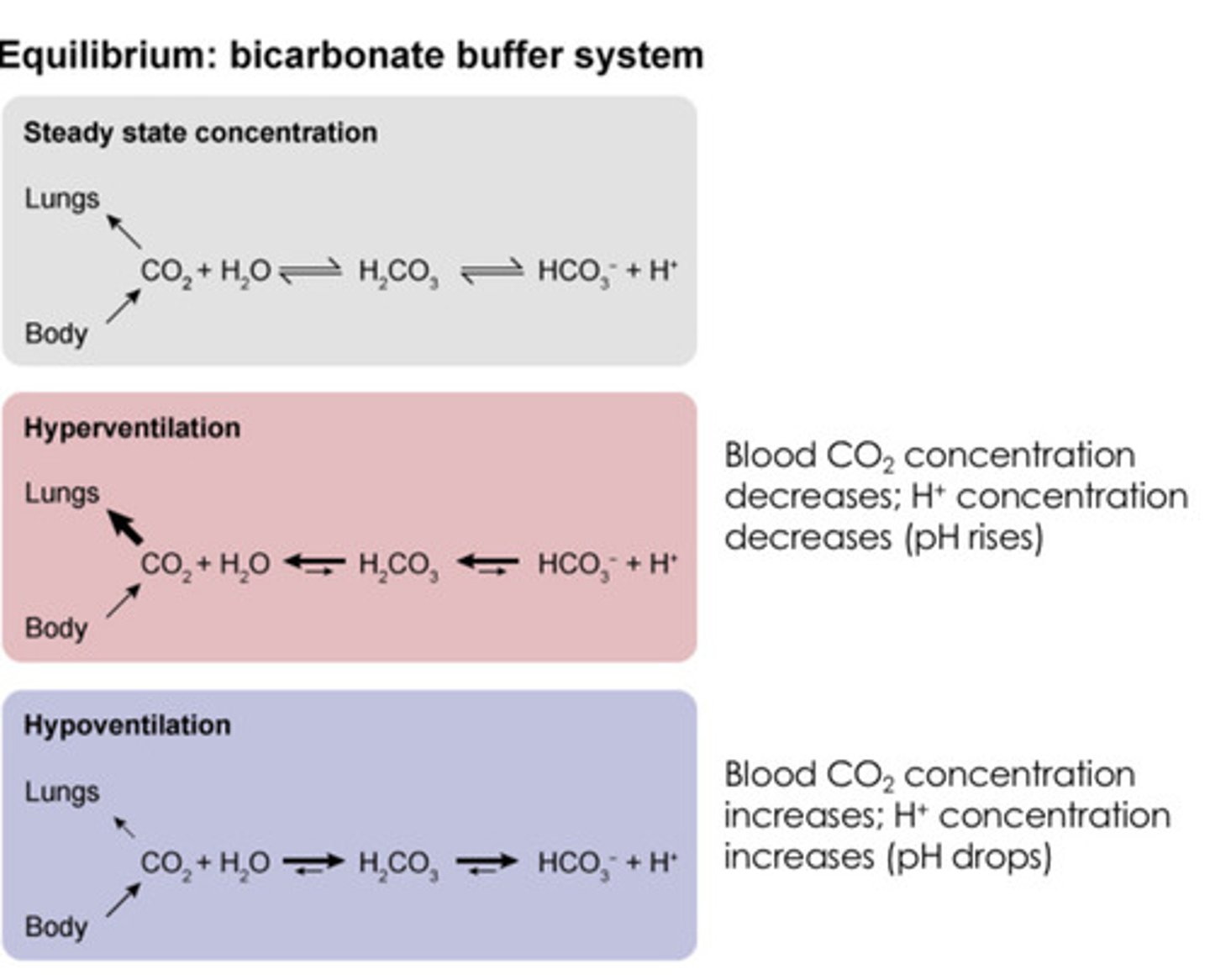
What would occur to the bicarbonate system if a pt is hyperventilating due to stress?
Co2 + H2O <-> H2Co3 <-> HCO3- + H+
Co2 levels drop due to less carbon dioxide being retained in the body (hyperventilation)
Co2 + H2O <-> H2Co3 <-> HCO3- + H+
H2Co3 levels drop due to less carbon dioxide being retained in the body
Co2 + H2O <-> H2Co3 <-> HCO3 + H+
Bicarbonate and hydrogen levels drop due to lower H2Co3 levels
Co2 + H2O <-> H2Co3 <-> HCO3 + H+
Since H+ levels drop, pH becomes more basic (higher pH)
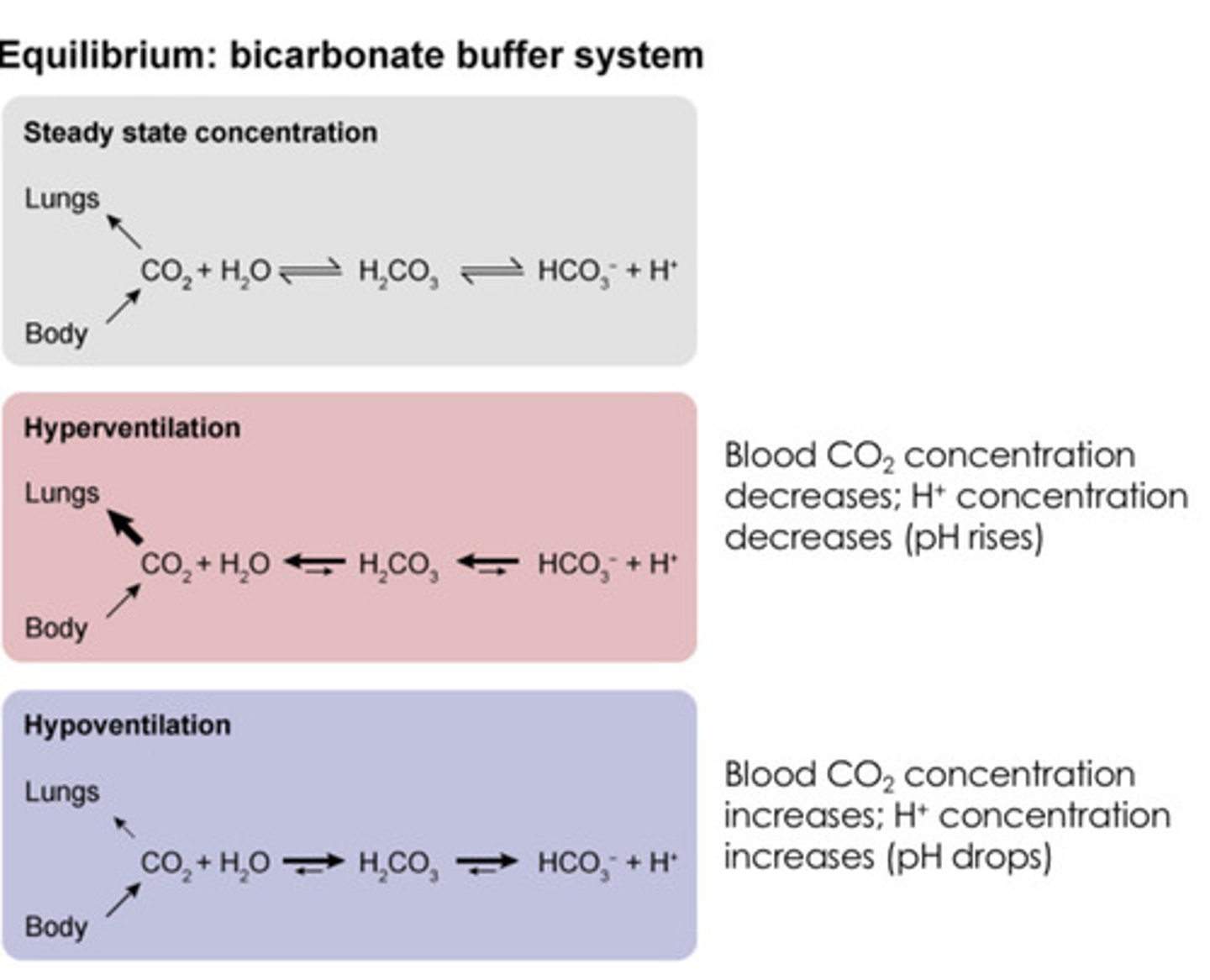
What would occur to the bicarbonate system if a pt is hypoventilating?
Co2 + H2O <-> H2Co3 <-> HCO3- + H+
Co2 levels increase due to more carbon dioxide being retained in the body (hyperventilation)
Co2 + H2O <-> H2Co3 <-> HCO3- + H+
H2Co3 levels increase due to more carbon dioxide being retained in the body
Co2 + H2O <-> H2Co3 <-> HCO3 + H+
Bicarbonate and hydrogen levels increase due to higher H2Co3 levels
Co2 + H2O <-> H2Co3 <-> HCO3 + H+
Since H+ levels increase, pH becomes more acidic (lower pH)
A patient with diabetes misses their insulin dose, resulting in the production of excess ketones, which results in acidosis? What is the bicarbonate system's method of compensating? (CS)
Metabolic Compensation
-More H+ ions are secreted through urine, resulting in the pH returning to neutral
Respiratory Compensation
-More CO2 is expelled via the lungs, resulting in less carbonic acid being produced and thus less H+ being produced, resulting in the pH returning to neutral