Chapter 22 Reaction mechanisms
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
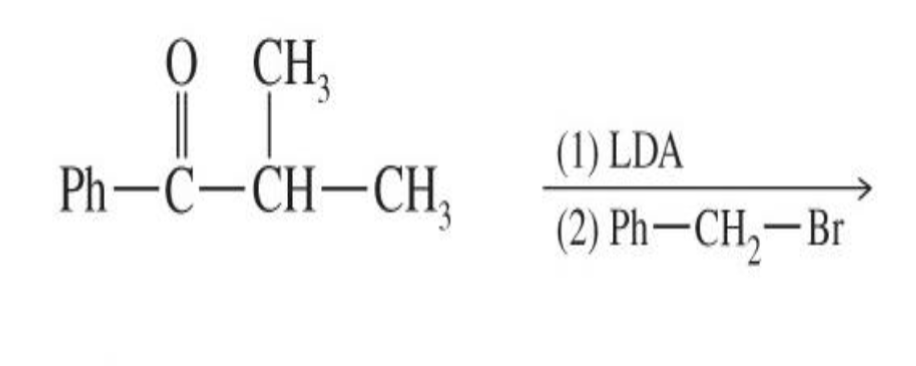
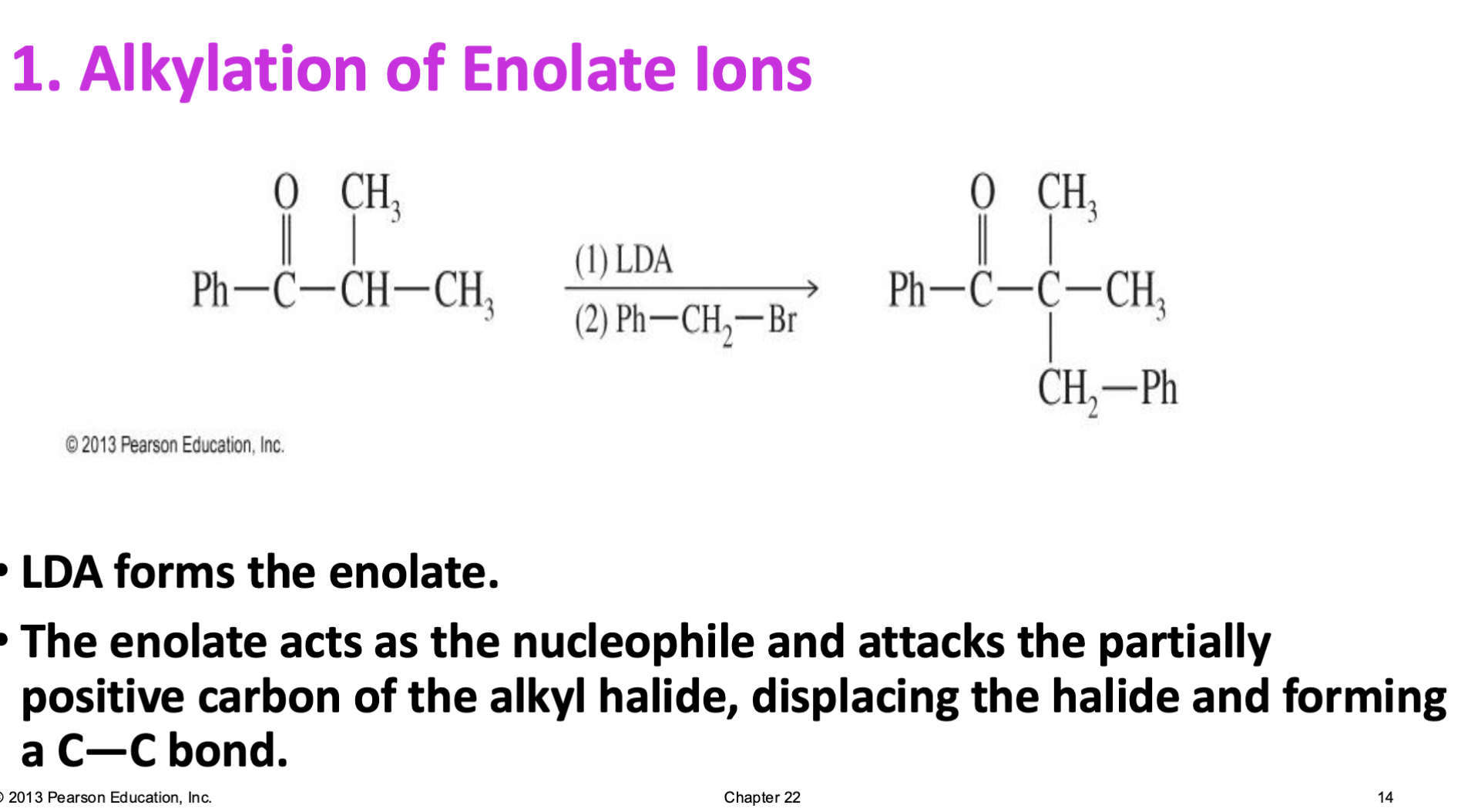
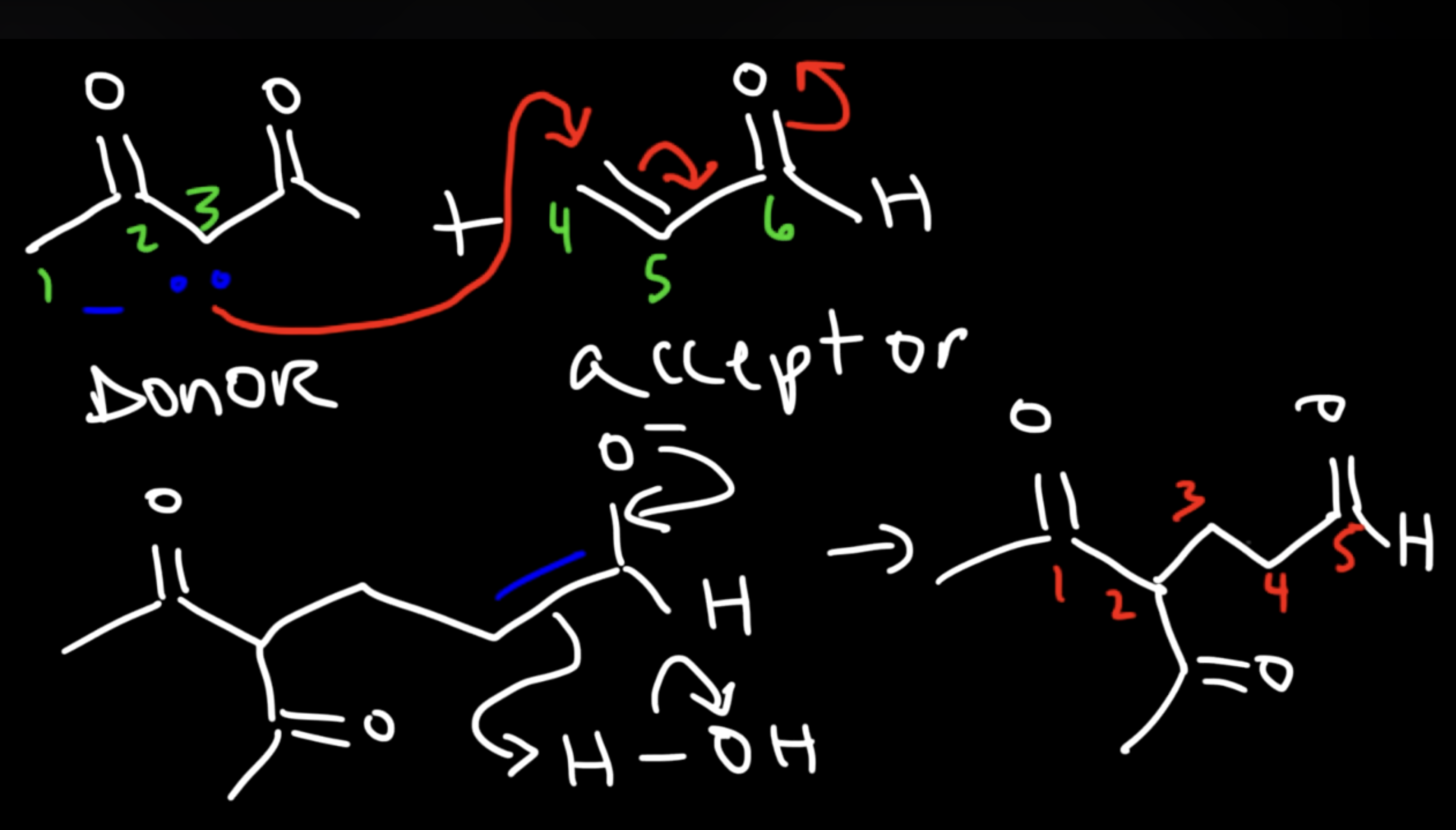
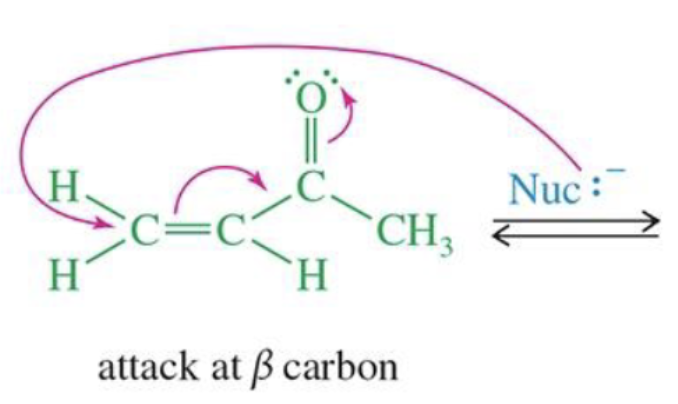
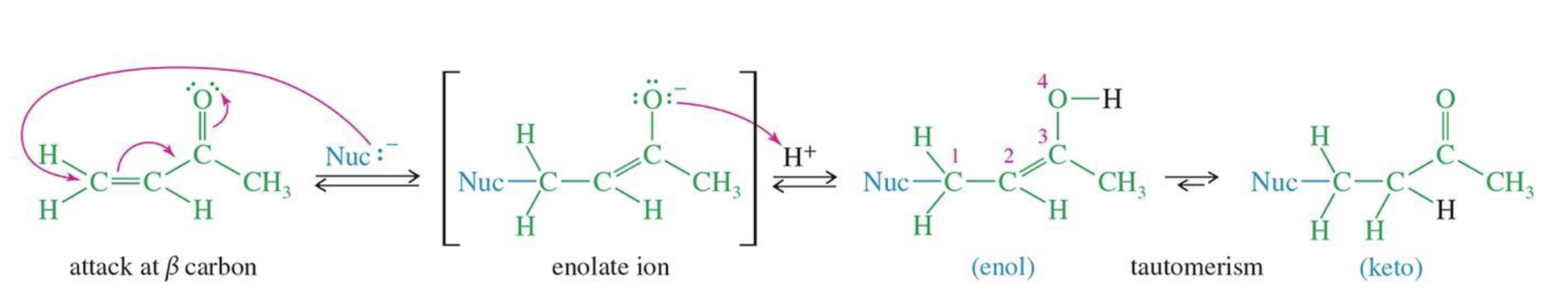
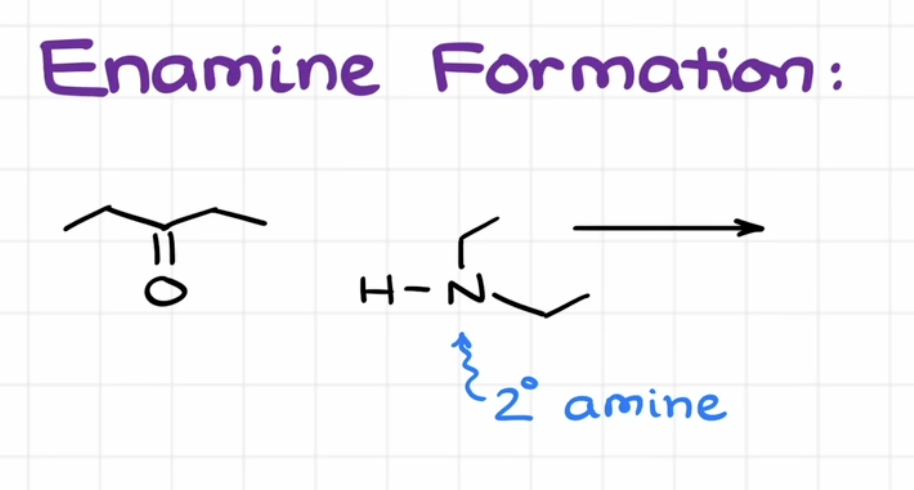
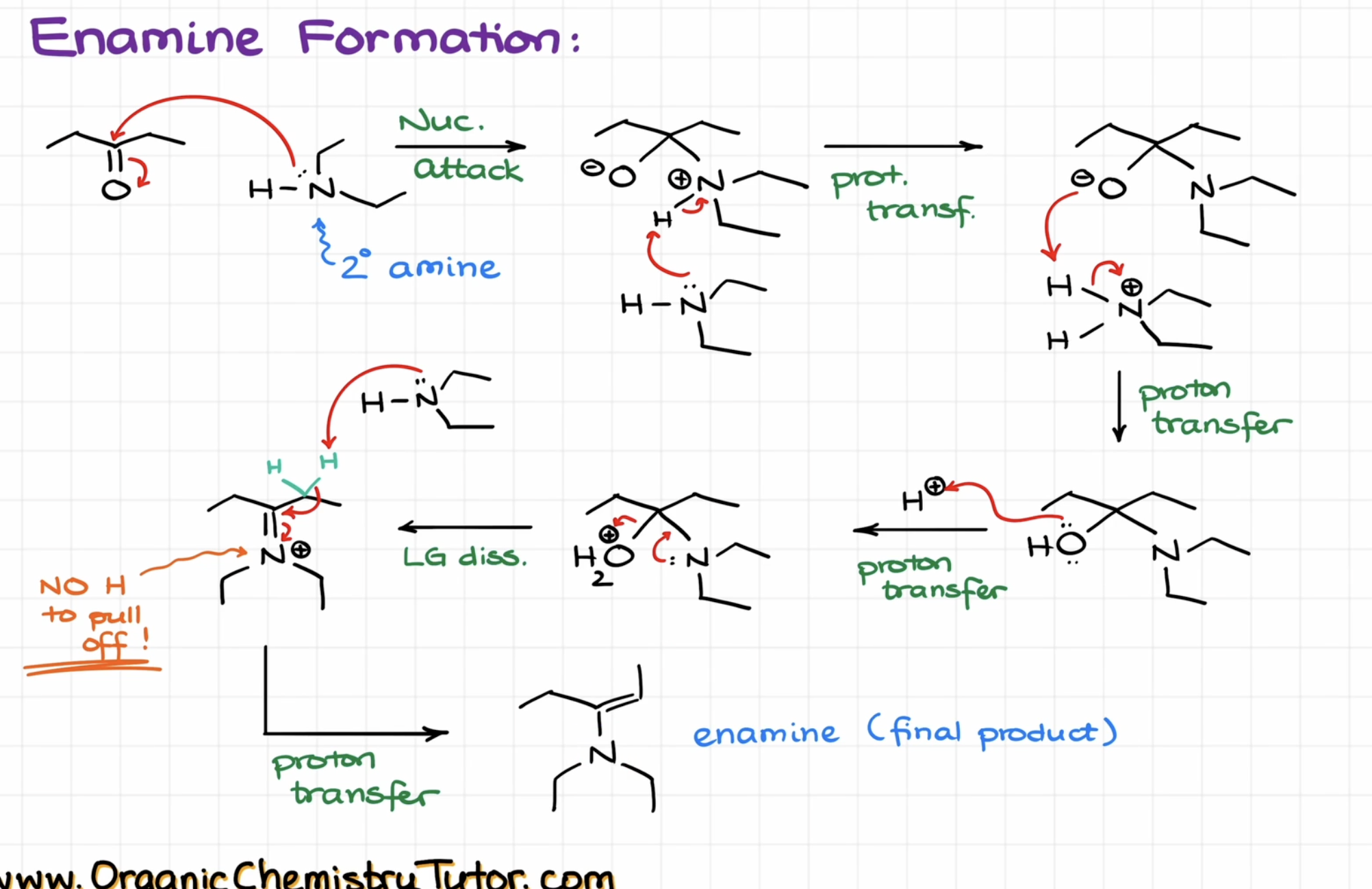
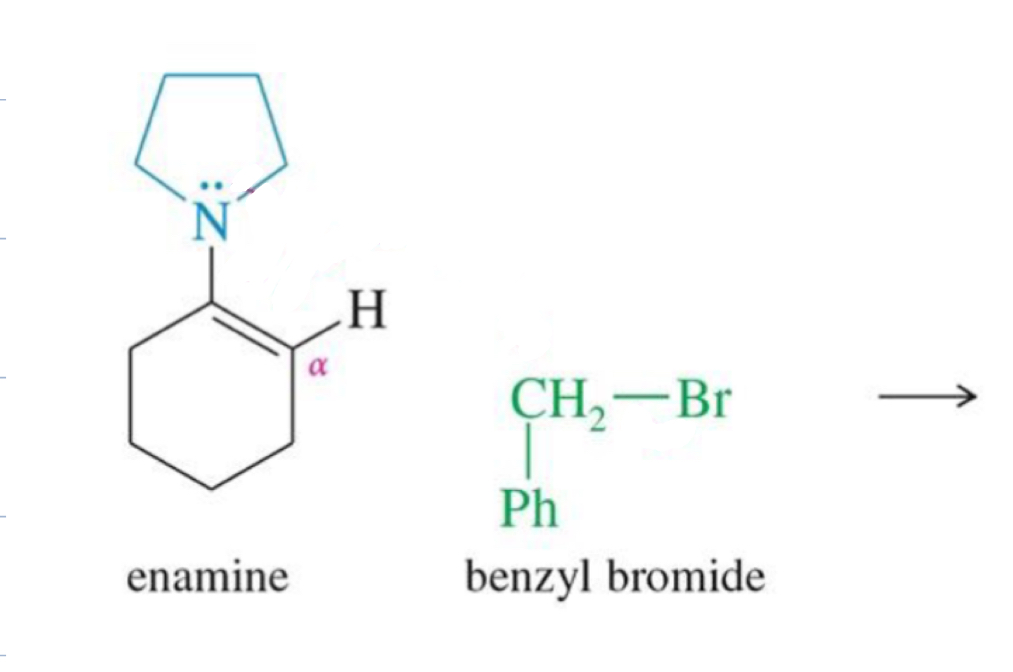
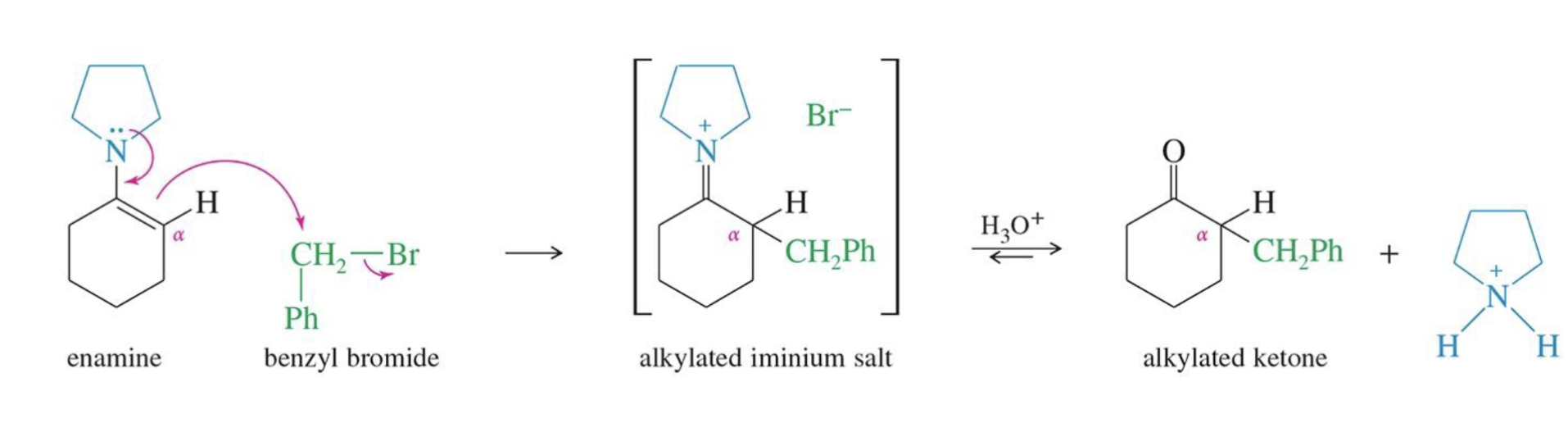
Enamine Formation

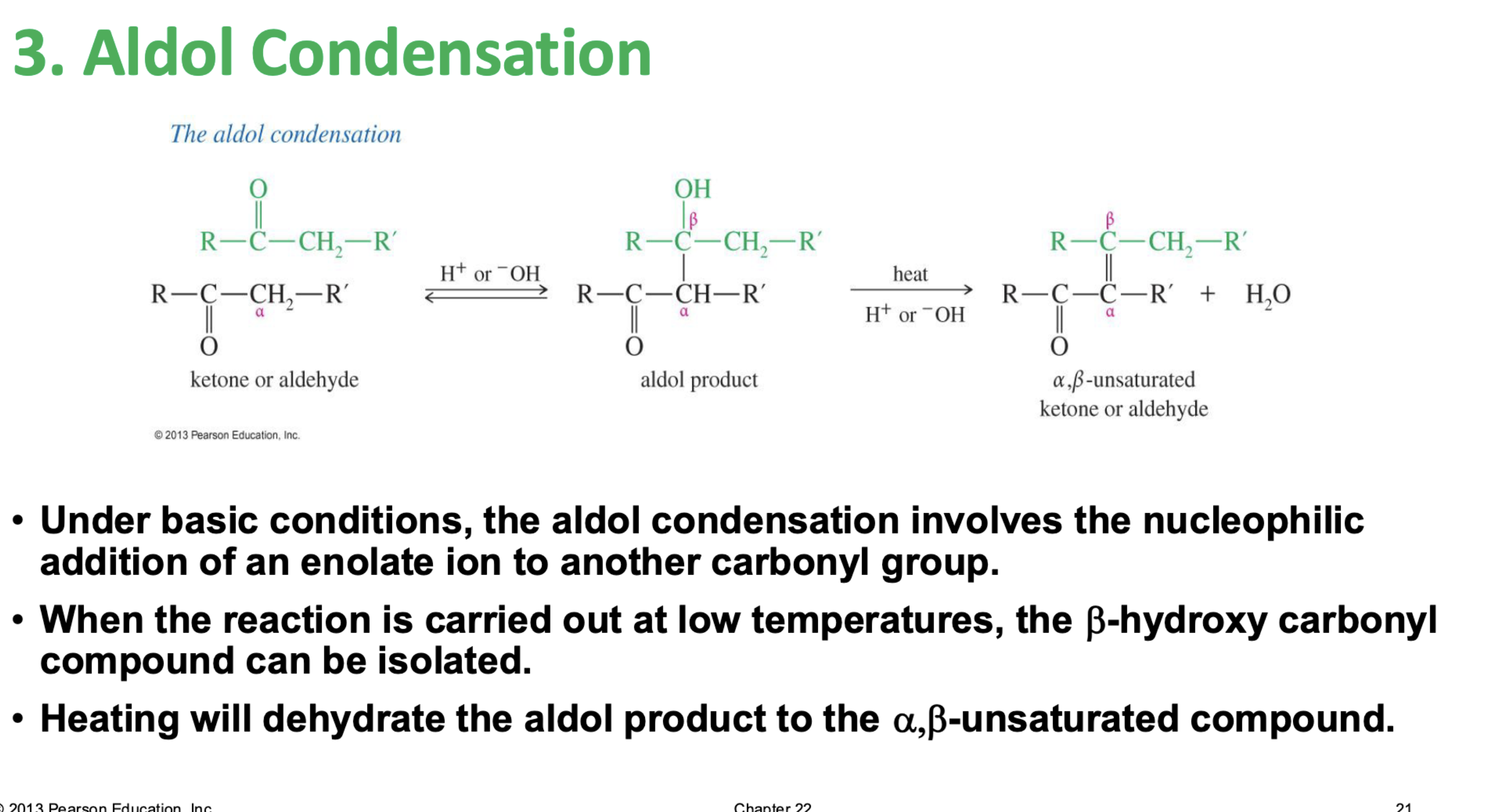
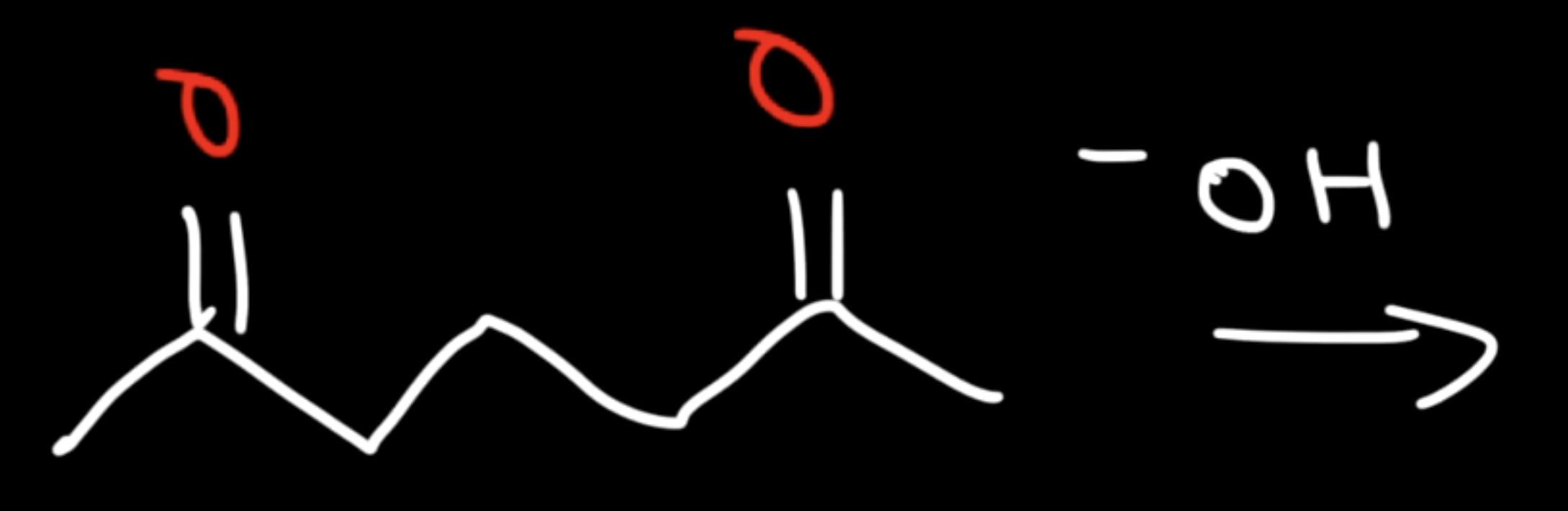
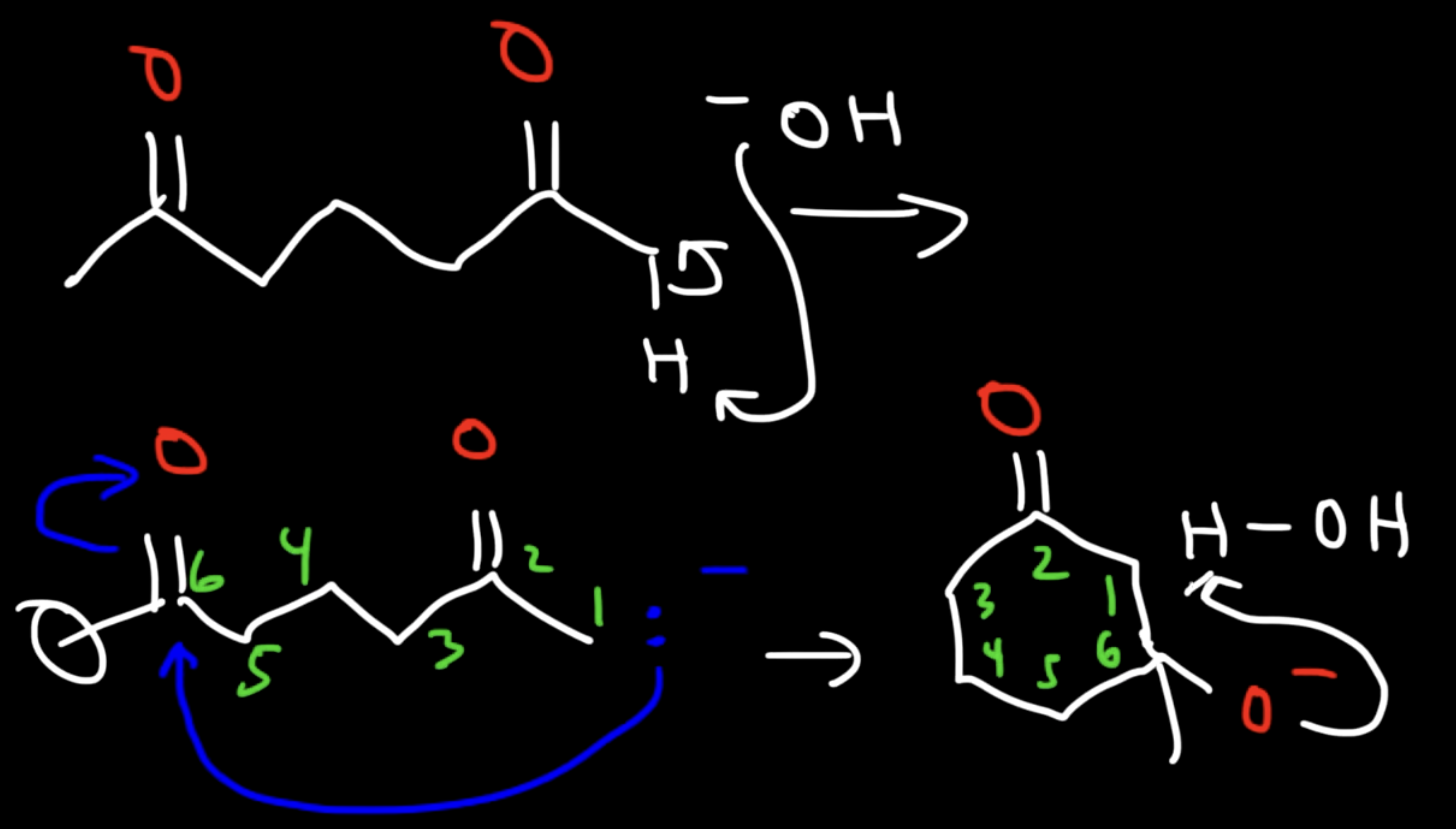
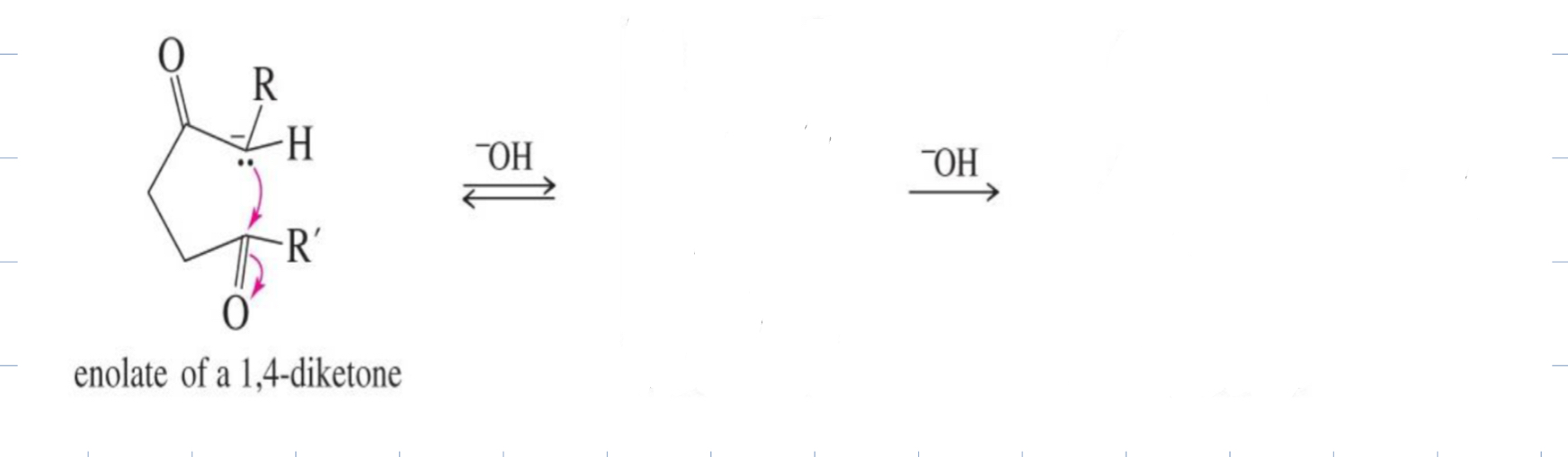
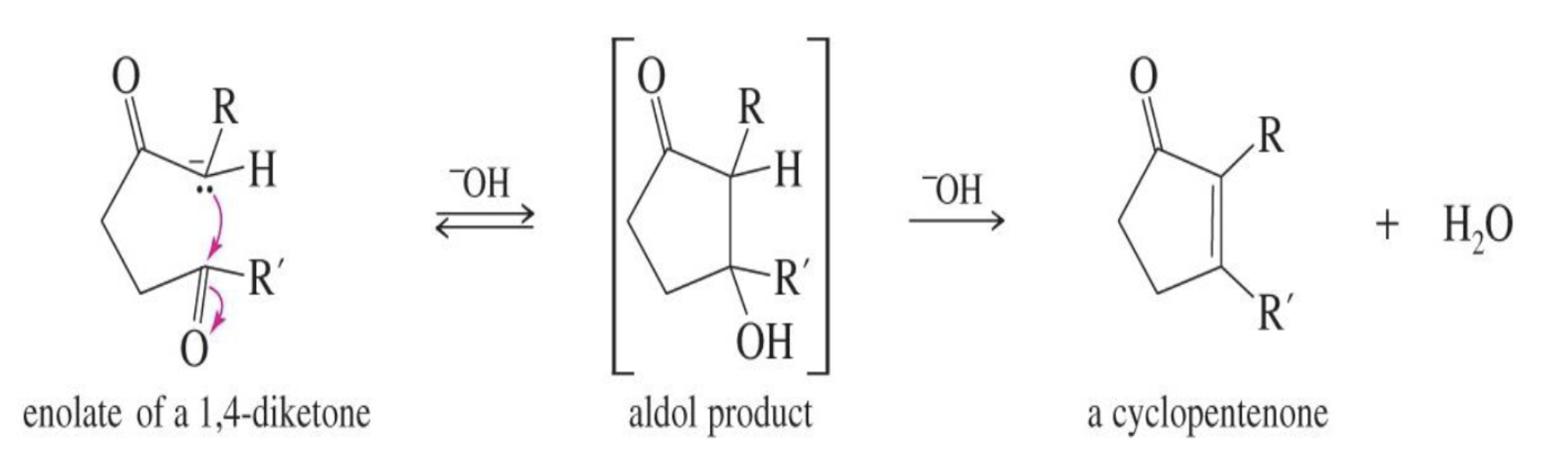
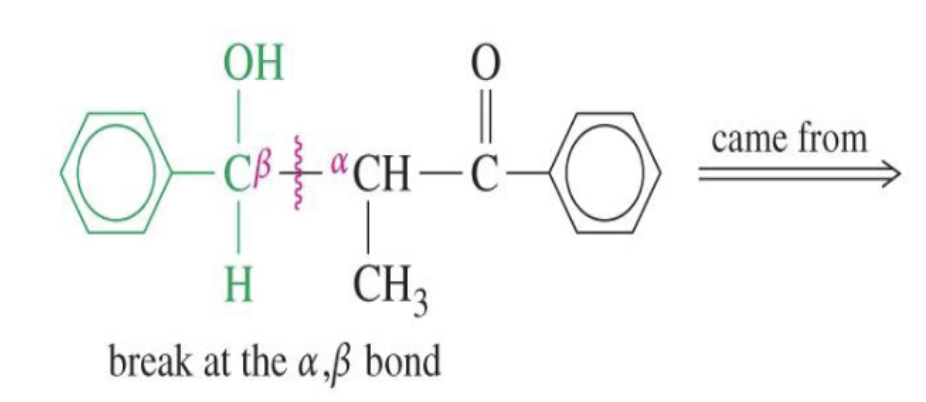
Base Catalyzed Adol Condensation
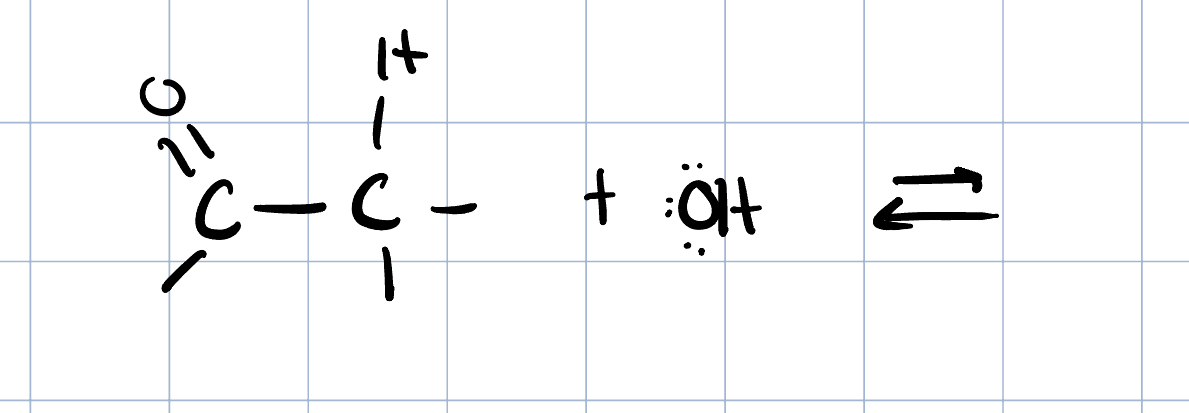
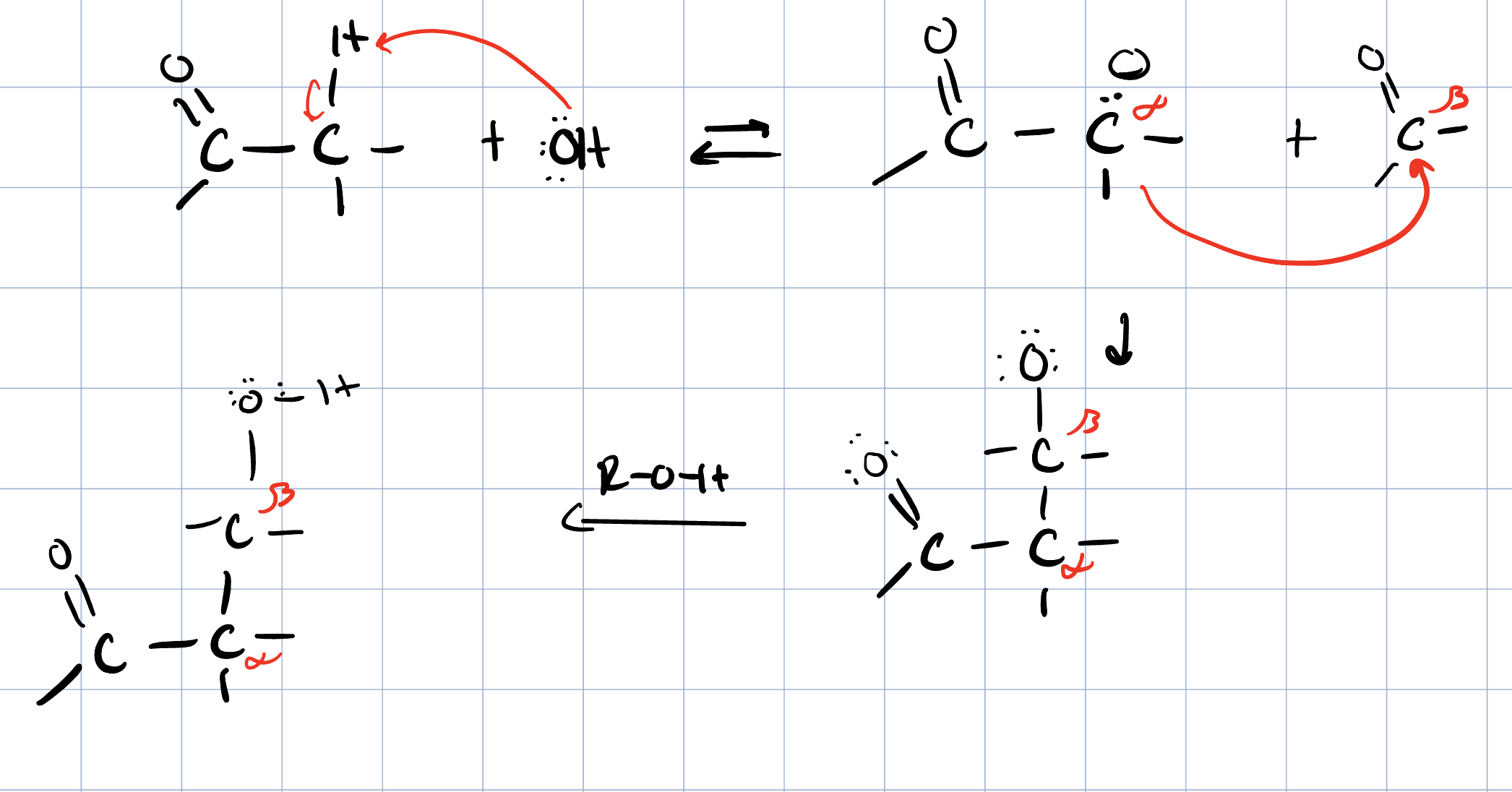
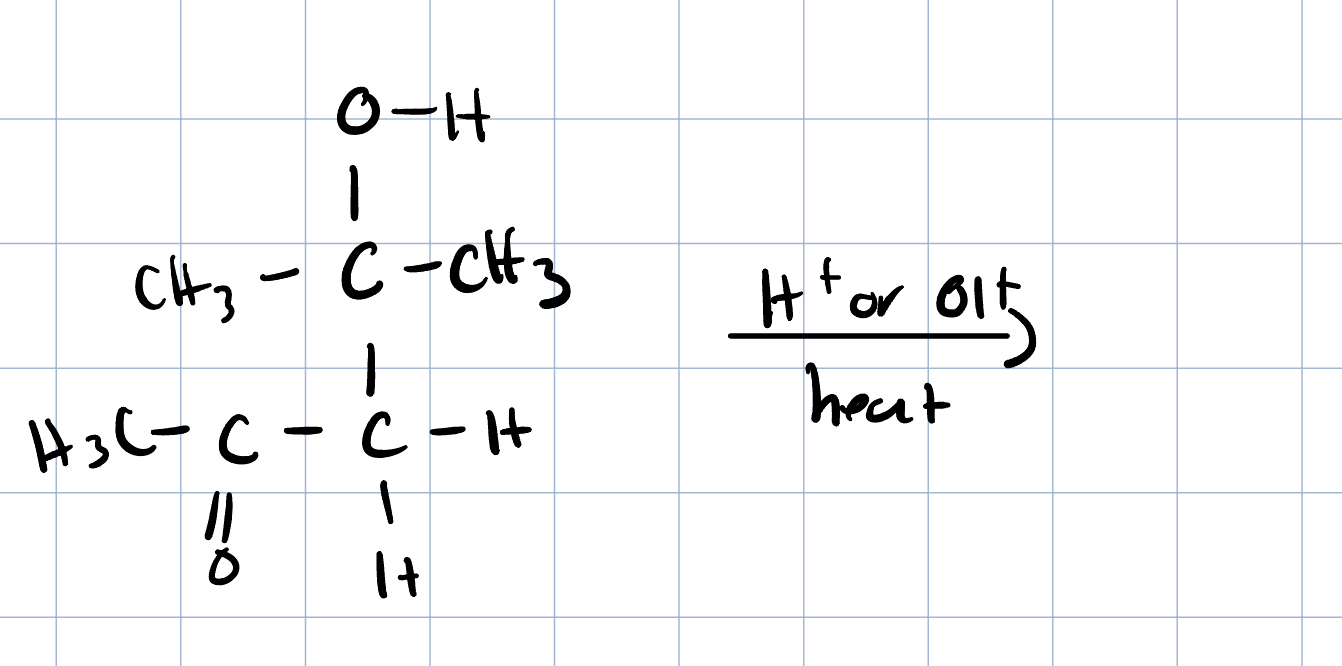
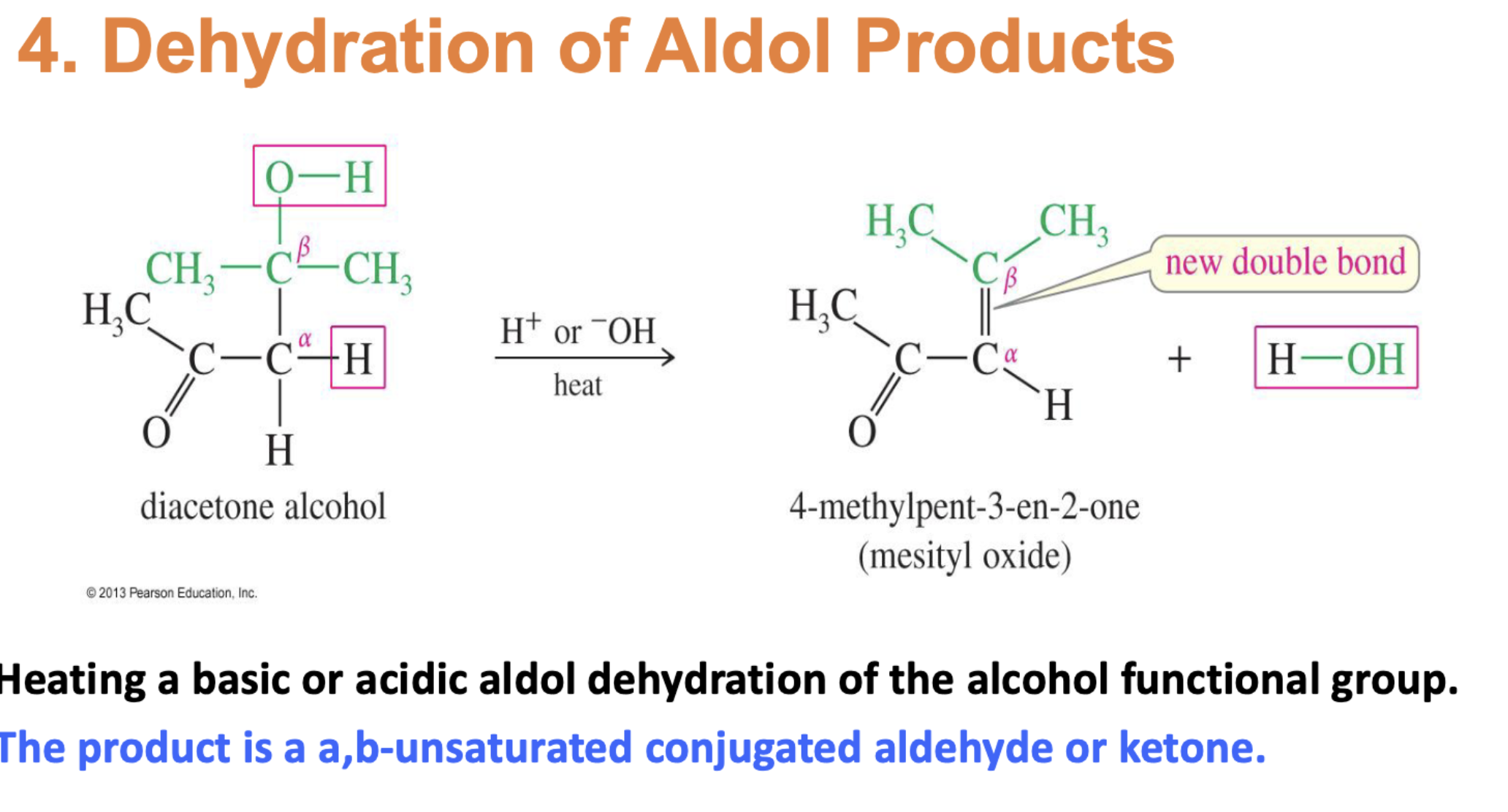
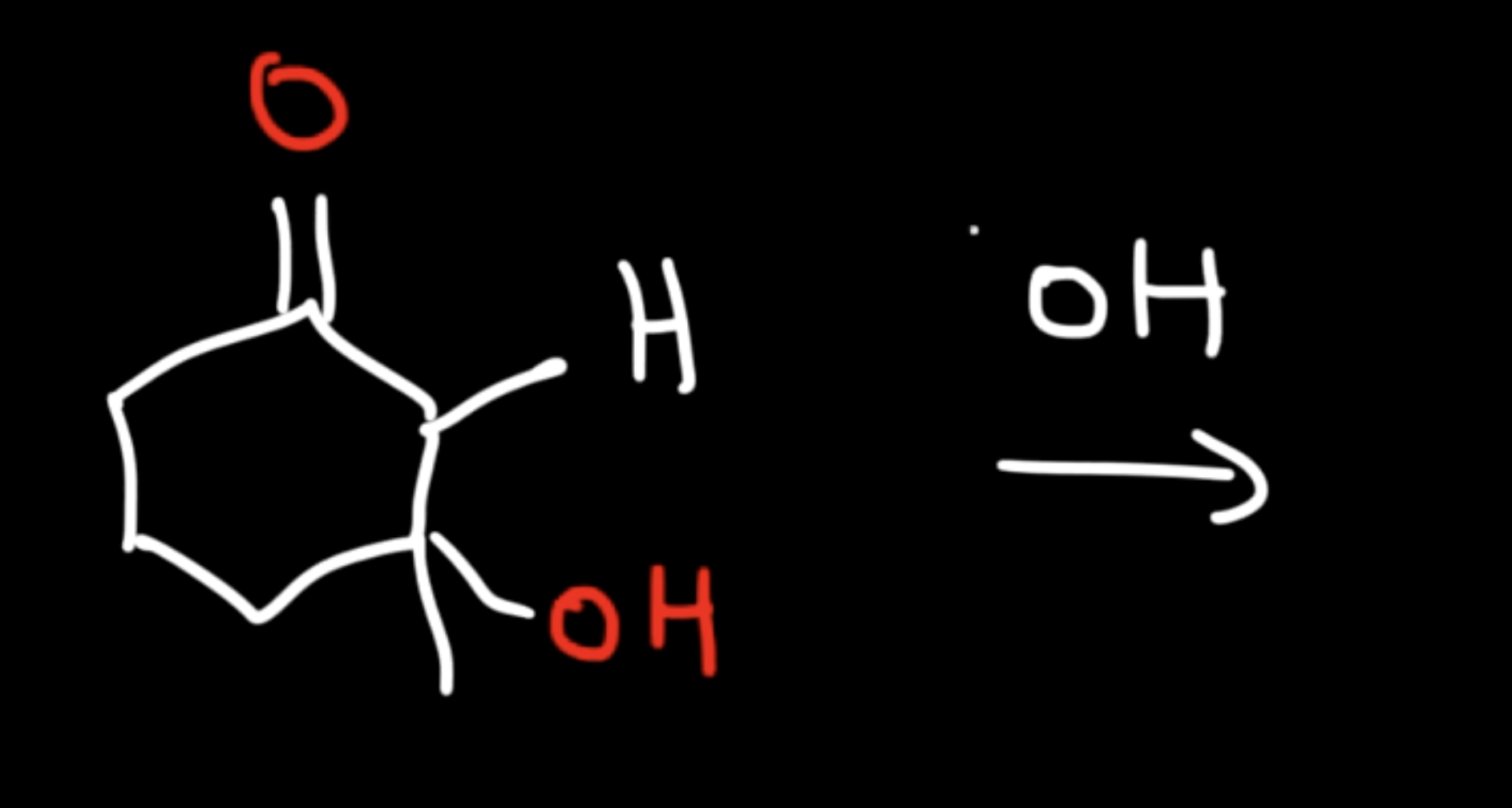
Saturated Product (Aldol Addition)
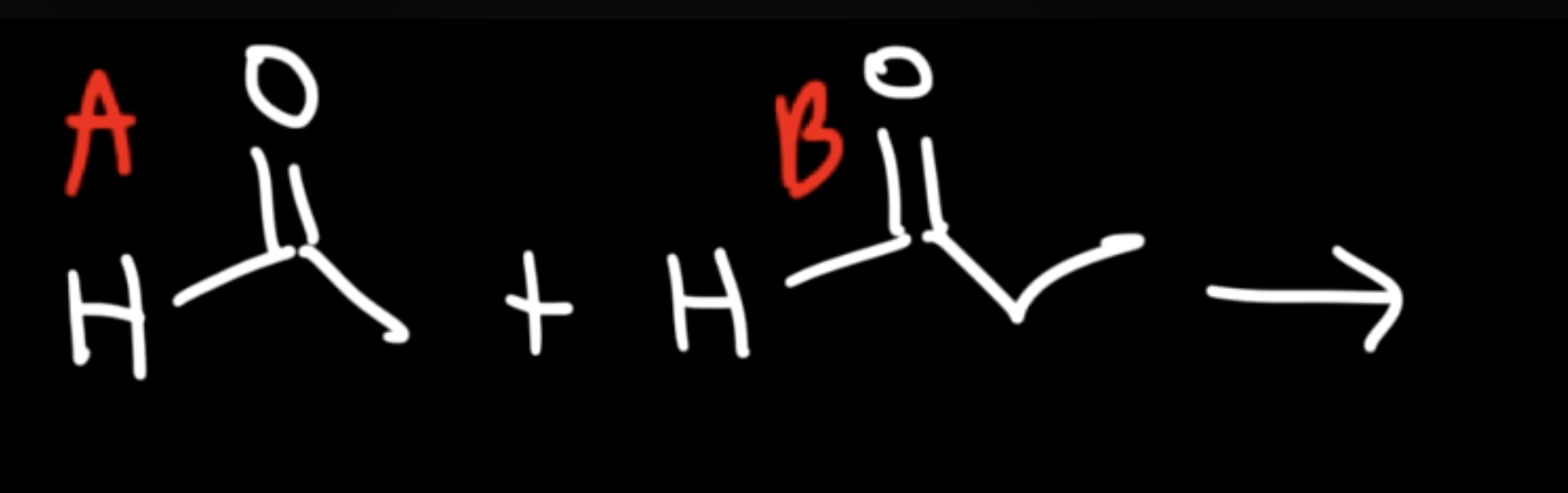
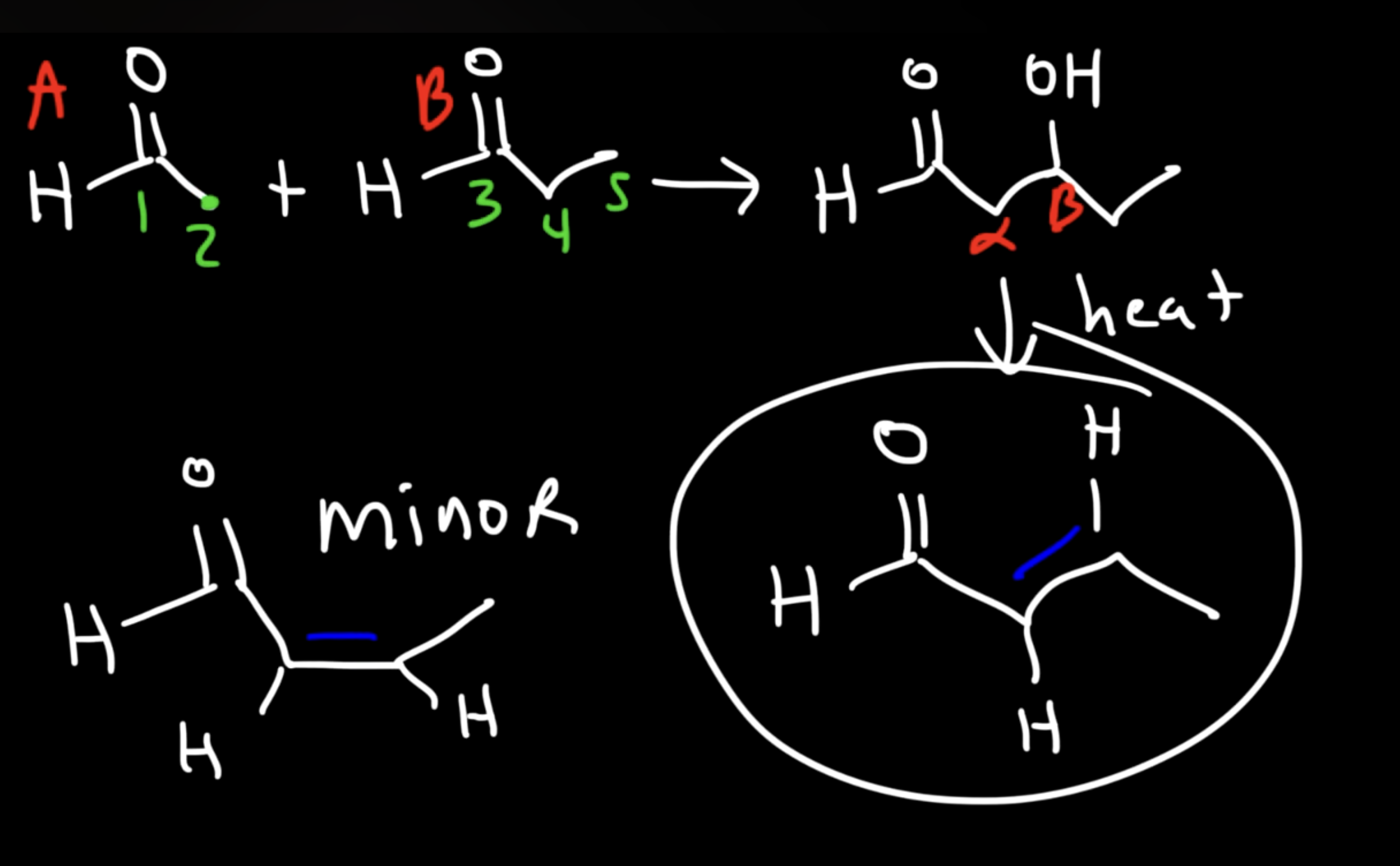
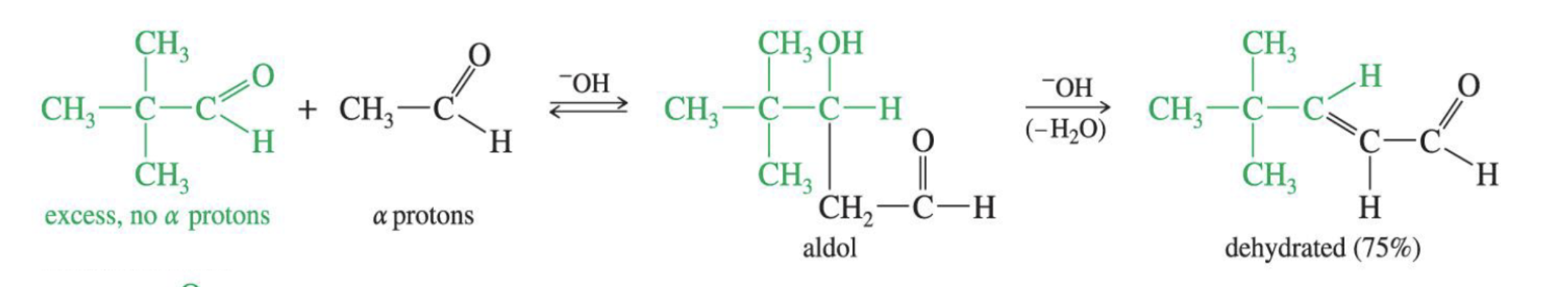
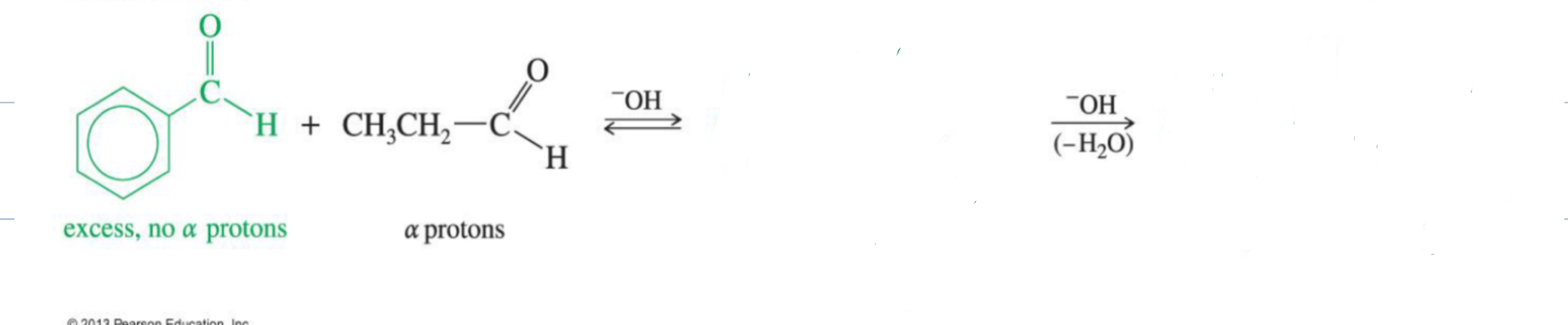
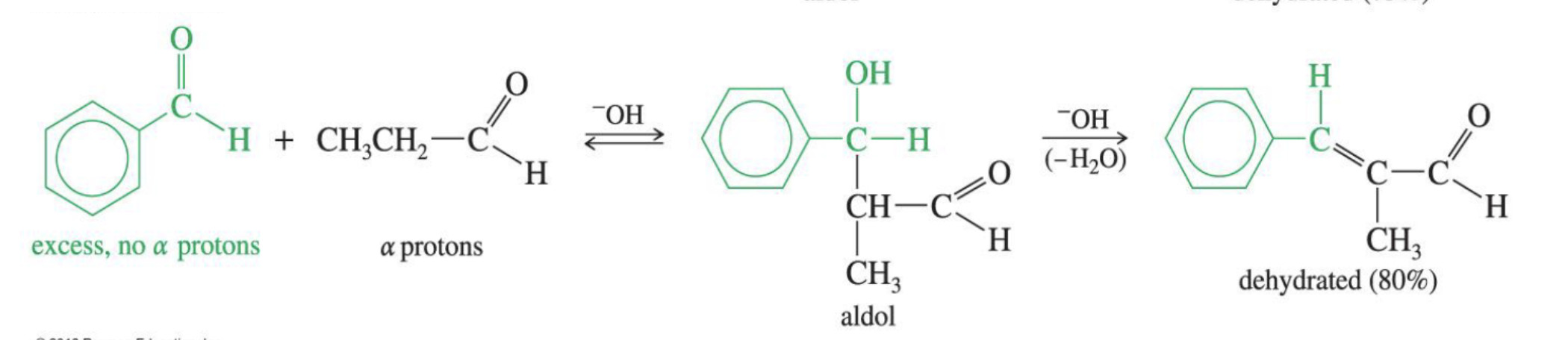
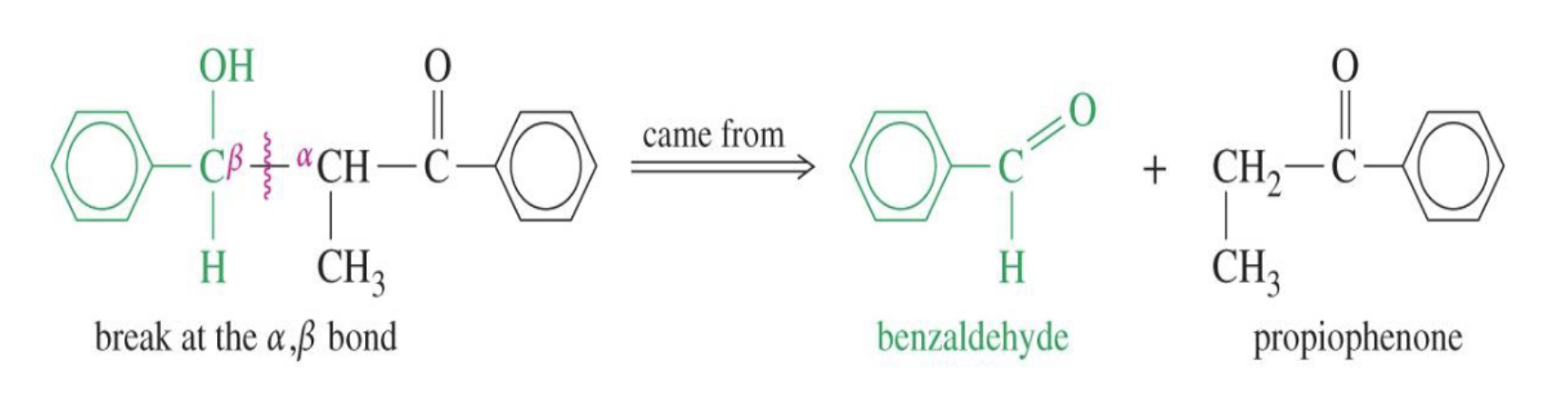
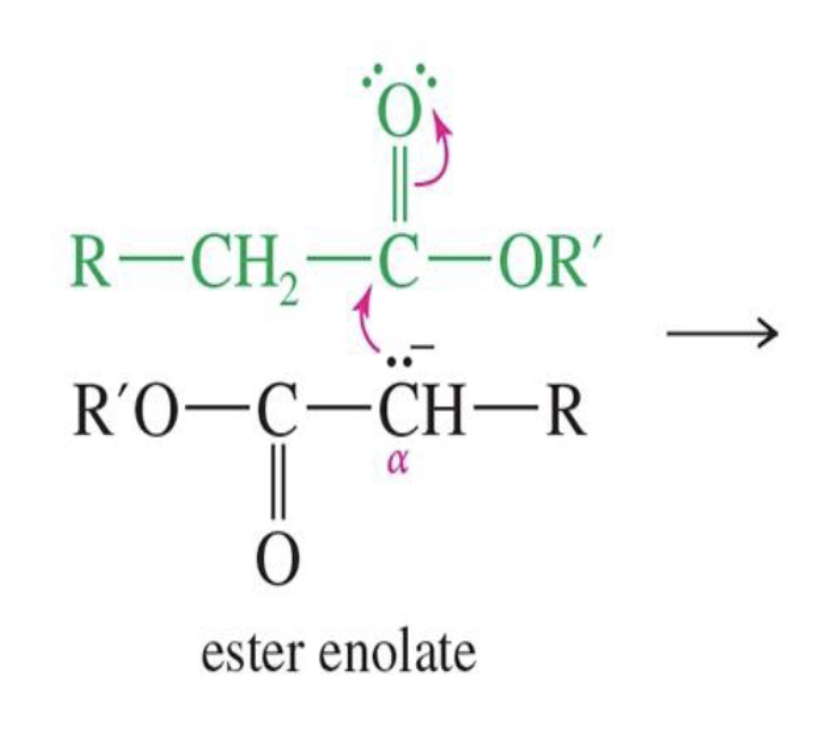
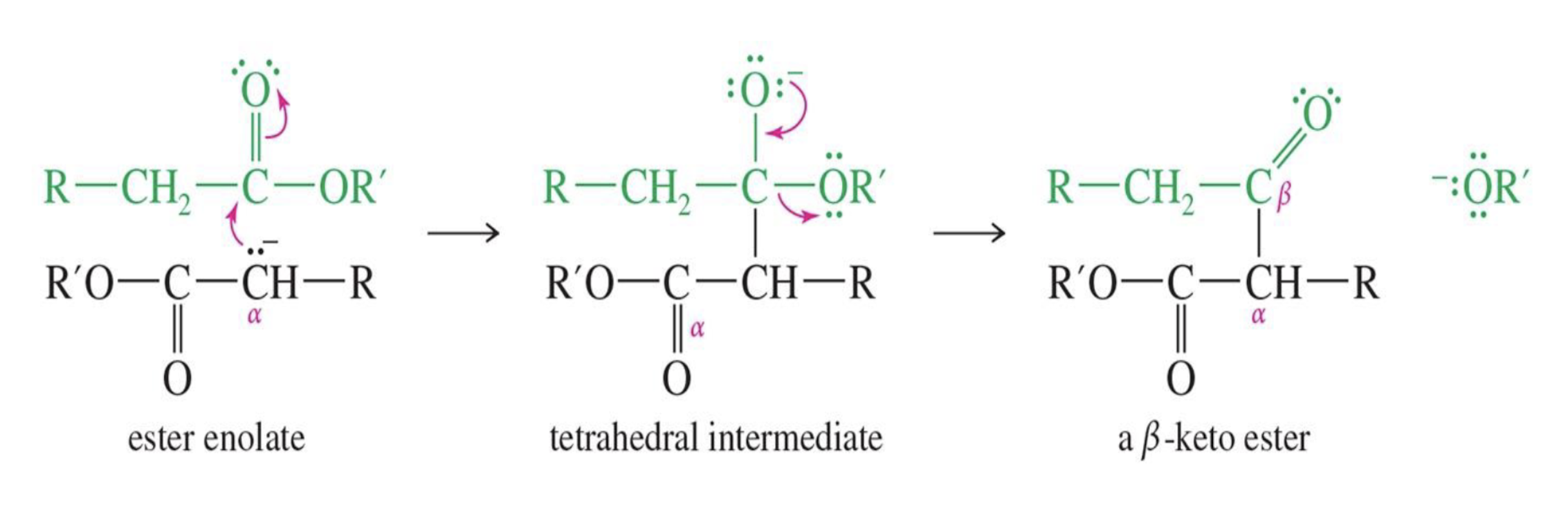
Reaction: The initial reaction between two aldehyde or ketone molecules (one acting as an enolate nucleophile, the other as a carbonyl electrophile) produces a beta-hydroxy aldehyde or beta-hydroxy ketone (an "aldol" or "ketol").
Saturation: The product is considered saturated because it only contains single bonds between the beta and alpha carbons. The carbon skeleton is fully "saturated" with the maximum number of hydrogen atoms or other groups in those positions.
Conditions: This intermediate is obtained by performing the reaction at low temperatures to favor the addition product and prevent the subsequent elimination reaction.
Reversibility: The aldol addition reaction is generally reversible, especially for ketones.
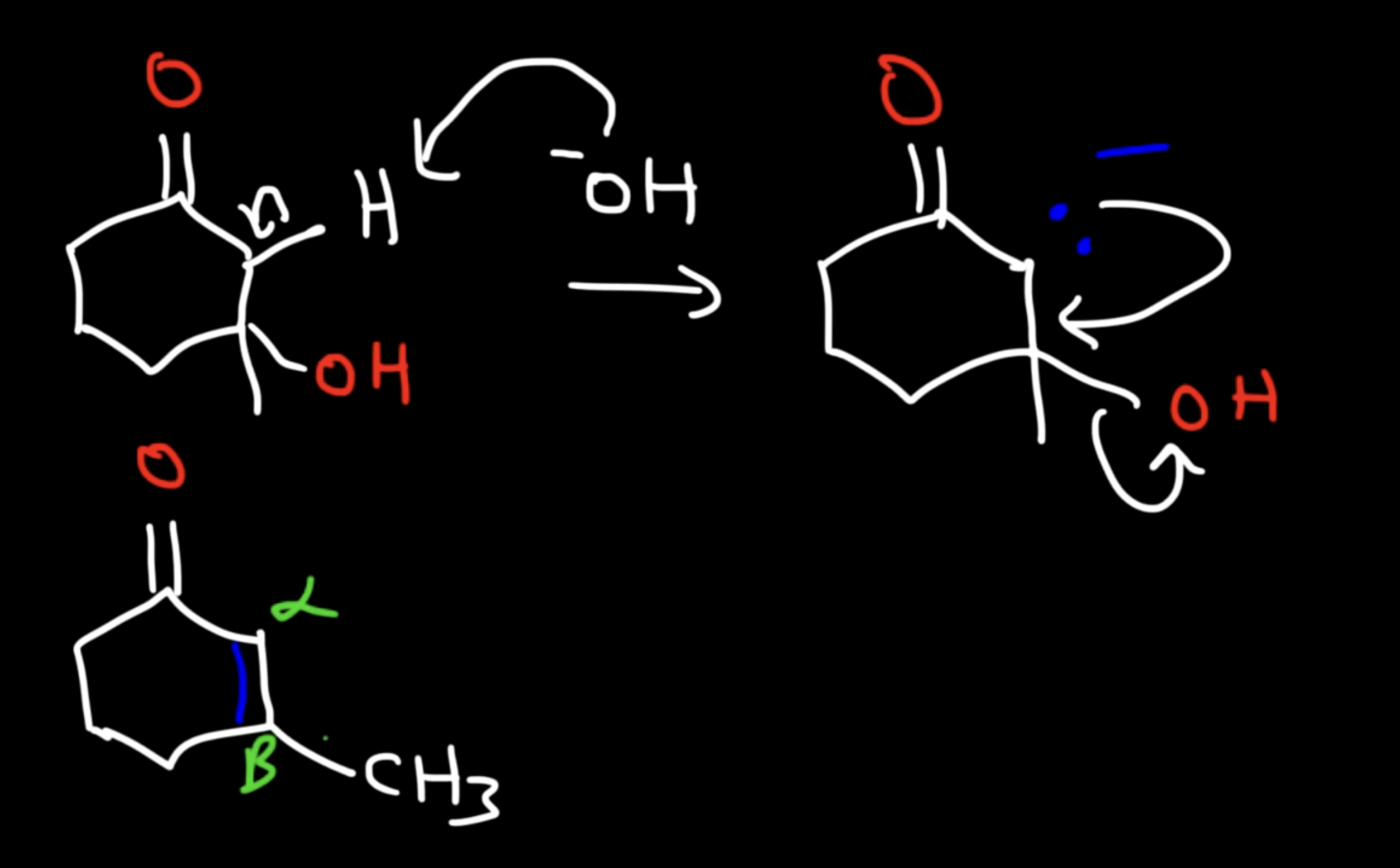
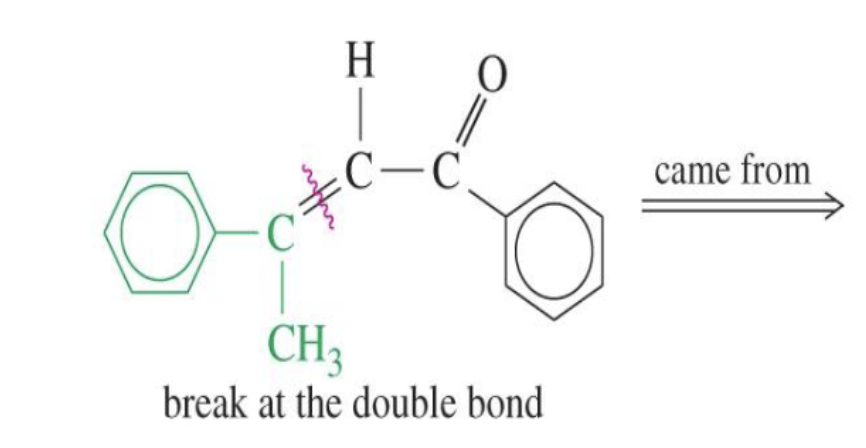
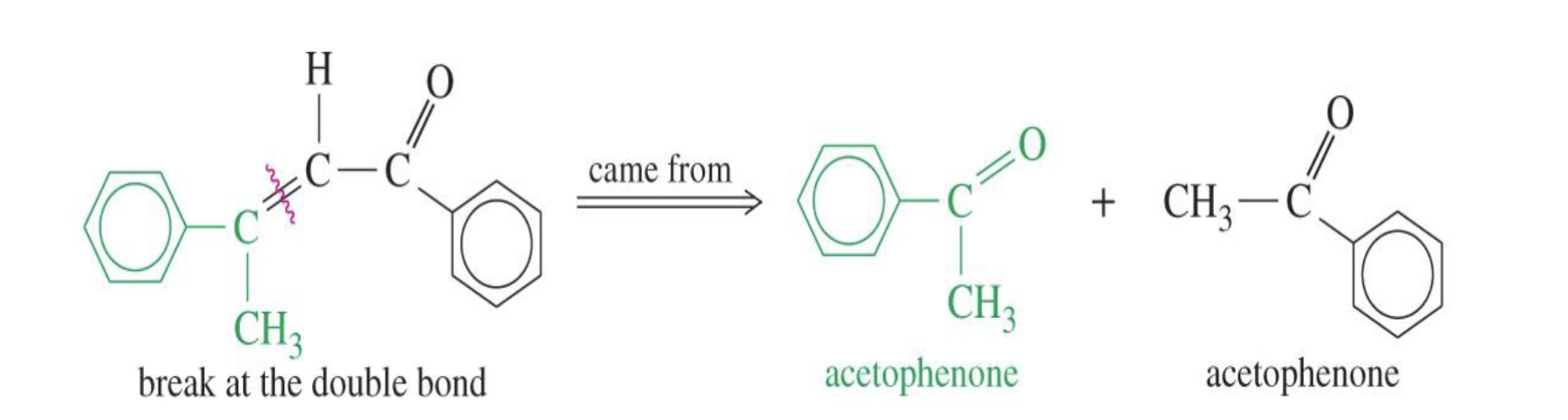
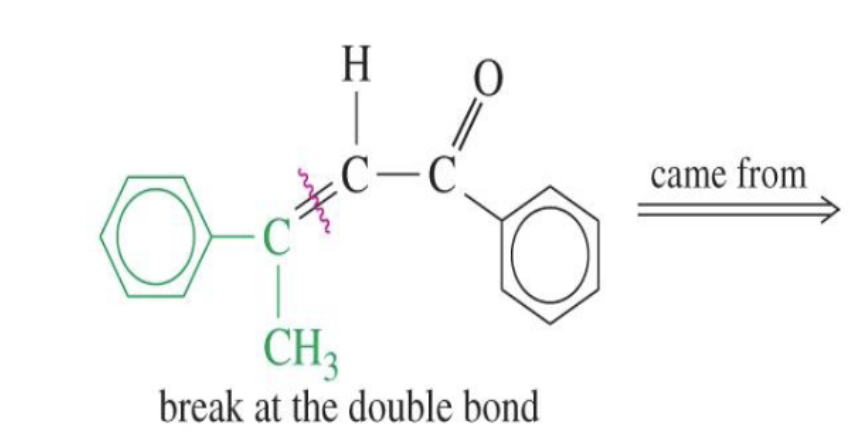
Unsaturated Product (Aldol Condensation)
Reaction: When the initial aldol addition product is heated (often in the presence of acid or base catalyst), it undergoes an elimination reaction (specifically, a dehydration) to remove a water molecule.
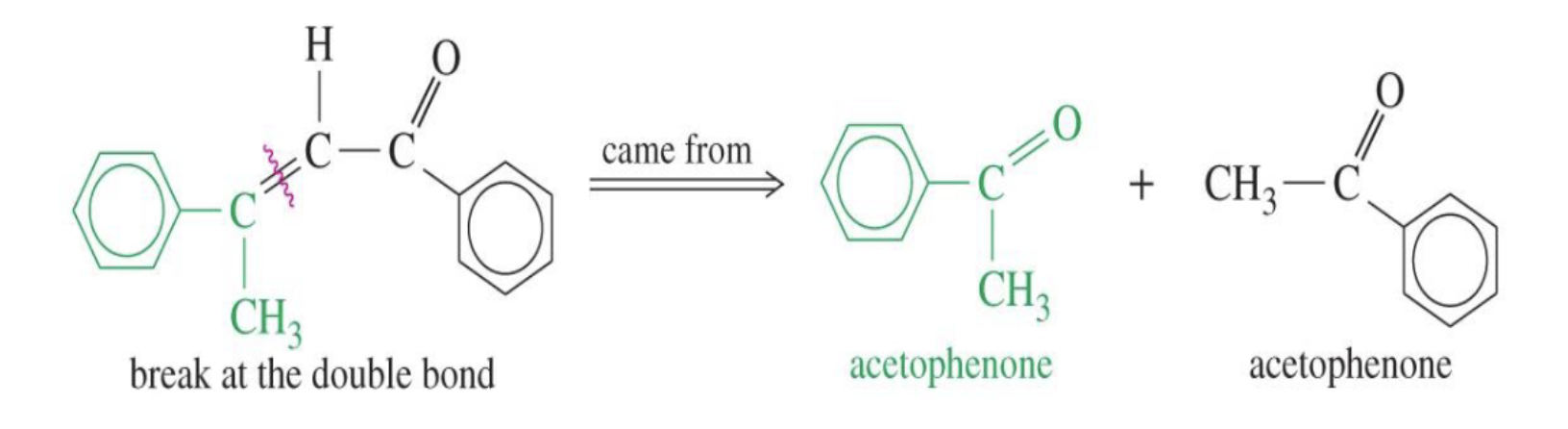
Unsaturation: This dehydration step introduces a carbon-carbon double bond between the alpha and beta carbons, resulting in an alpha,beta-unsaturated aldehyde or alpha,beta-unsaturated ketone. The double bond is in conjugation with the carbonyl group, which provides extra stability and acts as the driving force for the elimination reaction.
Conditions: The application of heat is the key condition that drives the reaction to completion and leads to the condensation (unsaturated) product.
Irreversibility: The formation of the conjugated alpha,beta-unsaturated system is a highly favorable, irreversible process under these conditions.