OHS 422: L02: Substitution of Materials and Control Banding
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
71 Terms
What is green chemistry?
the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances
is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances
green chemistry
Green chemistry applies across the
life cycle of a chemical product, including its design, manufacture, use, and ultimate disposal.
What are the general rules associated with substitution?
1. Prefer non-hazardous chemicals
2. Accept less-hazardous chemicals
3. Mission objective should be met
4. Consider physical, chemical, toxicological, and physiological characteristics
5. Consider sustainable outcomes
6. Consider green chemistry principles
If we are just comparying OELs to determine what chemical to select then we should select the one with the
greatest limit
What are the limitations of just using the OEL to determine if it should be selected?
1. Volatility not considered
2. Limits may change with time
3. Limits do not represent a clear line between safe and unsafe conditions
4. Mainly inhalation is considered
5. Many chemicals do not have OEL
ACGIH TLV Disclaimer says you should
not use them as a relative indicator of toxicity
ACGIH TLV Disclaimer says you should not use them as a relative indicator of toxicity
TLVs are designed to
protect against a specific "critical effect"
Which chemical should we select?
chemical A

Which chemical should we select?
chemical B
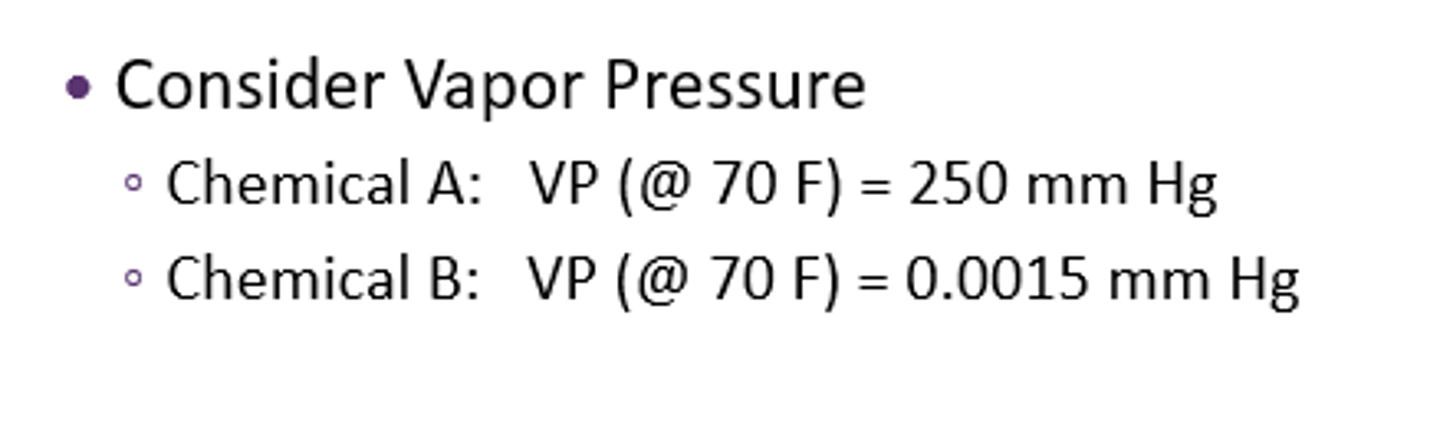
the pressure exerted by a vapor/gas in equilibrium with its liquid phase at a given temperature, in a closed system
vapor pressure
Define vapor pressure.
the pressure exerted by a vapor/gas in equilibrium with its liquid phase at a given temperature, in a closed system
the tendency of particles to escape from the liquid
vapor pressure
the higher the VP of a substance at a given temperature, the
higher the volatility and the higher the concentration of the substance in the air
If we are just comparying vapor pressures to determine what chemical to select then we should select the one with the
lowest vapor pressure
Lower vapor pressures mean that it is
less volatile
What are three methods we can use to select chemicals that are the least dangerous ?
1. vaphor hazard ratio
2. dilution factor
3. toxicological data
What is the vapor hazard ratio?
A unitless number that takes into account (1) the toxicity of the chemical that is based on the OEL and the volatilty of the chemical
A unitless number that takes into account (1) the toxicity of the chemical that is based on the OEL and the volatilty of the chemical
vapor hazard ratio
When using the VHR, Ceq is the
equilibrium concentration in ppm
When calculating Ceq, the VP is in
mmHg
When using VHR, which chemical should we select?
the one with the lowest VHR
What are the problems associated with using the VHR?
1. Limitations of the OELs
2. Estimates of equilibrium concentration (Ceq) are different depending if the process allows molecular or eddy diffusion
3. Issues with mixtures of liquids
What is eddy diffusion?
you are promoting the evaporation process (i.e. you are forcing the chemical to evaporate)
The VHR is a
worst-case scenario because it is calculated right above the surface with no dilution
What are some of the limitations with the OEL?
there may not be one
which OEL do we use?
the OEL is not a safe line
What is molecular diffusion?
diffusion from high to low concentrations with no competing forces
When using the dilution factor, you should select the chemical with the
lowest DF
We should select the chemical agent with lowest DF. Why?
it means less air is needed to dilute it to a concentration under the OEL
What is the dilution factor?
the number of m3 of fresh air needed to dilute 1 liter of vapor of the contaminant below the TLV
the number of m3 of fresh air needed to dilute 1 liter of vapor of the contaminant below the TLV
dilution factor
What are the limits with use toxicological data?
we need to be comparing similar effects in similar animals
What are the two ways to find VHR when dealing with a mixture?
ideal or nonideal behavior
What is an ideal gas?
hypothetic gas whose molecules themselves take up no volume and do not interact with each other.... Thus they obey the gas laws exactly.
If it's an ideal mixture, we know from chemistry the mixture will
obey Raoult's Law
What is Raoult's law?
The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure component at that temperature multiplied by its mole fraction in the mixture (i.e. molar fraction in the liquid phase).
The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure component at that temperature multiplied by its mole fraction in the mixture (i.e. molar fraction in the liquid phase).
Raoult's Law
What is the formula for Raoult's Law?
Partial VP = (mole fraction) x VP
the molar fraction used in the VHR calculation of ideal mixtures is
the molar fraction in the liquid phase
How do you calculate molar fraction is the % by volume is known?
1. Assume 100 mL of liquid
2. Obtain the mL of each component
3. Convert mL into grams (use liquid density)
4. Convert grams to moles (use MW)
5. Calculate the molar fraction of each component
when finding VHR for a non-ideal mixture you must consider the
coefficient of activity for each chemical in the mixture
What type of mixture will we always assume in industrial hygiene?
ideal
When does Control Banding (CB) become a viable strategy to control occupational exposures?
CB becomes a viable strategy for controlling occupational exposures when there are no established occupational exposure limits (OELs). Without established OELs, employers and workers often lack the needed guidance on the extent to which occupational exposures should be controlled; control banding can serve as the solution for this.
What is the conceptual basis for CB?
The conceptual basis for CB is the grouping of chemical exposures according to similar physical and chemical characteristics, intended processes/handling, and anticipated exposure scenarios (amount of chemical used and how workers would be exposed).
One of the least complex models of CB includes a four-level hierarchy of risk management options. Which are these options?
1. Good occupational hygiene practices, which may be supplemented by use of appropriate personal protective equipment (PPE)
2. Engineering controls, including local exhaust ventilation (LEV)
3. Containment
4. Seeking specialist advice
Should CB be a considered an equivalent substitute to the Occupational Exposure Limits (OELs)?
CB is not meant to be a substitute for OELs, and the use of CB does not erase the need for environmental monitoring and industrial hygiene expertise. Labeling of chemicals in CB is a useful practice, but it is not intended to replace OELs, exposure assessment, or classic IH protocol.
Why is the reliance upon sampling and then comparing the sampling results with published OELs becoming more difficult?
Strict reliance upon sampling, analyzing airborne contaminants, and then comparing the results with OELs has become increasingly difficult in recent decades because of the growing number of hazardous chemicals. This increasing number far outweighs the ability and resources of agencies external to chemical manufacturers to develop OELs. If we do not have an established OEL, we cannot compare our sampling results with an OEL for that chemical making it hard to rely on OELs alone to determine safe levels.
What is expected that the regulation known as REACH accomplish in the European Union?
The European Commission promulgated regulations known as the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) to shift the burden of proof of chemical safety to manufacturers. This would apply to most chemicals in commerce.
What is the objective of COSHH? Where was this system developed?
Control of Substances Hazardous to Health (COSHH) is a simplified strategy to assess health risks in the workplace. It was developed in the United Kingdon (England) in the late 1980s to control workplace exposures to hazardous chemicals and is enforced by the United Kingdom Health and Safety Executive (HSE).
What control option is most commonly selected in England, even after the passage of COSHH?
Most respondents took measures to protect workers, mainly by making personal protective equipment available, followed by process controls.
What were the conclusions reached by Topping et al. concerning the OEL system?
Topping et al. concluded that, given the widespread lack of understanding about OELs, the creation of additional lists of OELs would not be cost effective and that OELs should be limited to widely used substances of concern. They also recognized that OELs and other information about the chemical (e.g., physical properties, use) could be used to recommend appropriate control measures. These authors suggested reappraisal of the traditional OEL system, which resulted in the birth of control banding.
Development and implementation of CB requires five actions. Which are these actions?
Development and implementation of a CB strategy requires five actions: creation of the strategy, its application, the installation and operation/maintenance of controls, postcontrol monitoring, and failure analyses at each step of the CB process.
Users of CB must have a complete understanding of R-phrases. What are R-phrases?
R-phrases are risk phrases. R-phrases describe the specific risks associated with a hazardous substance. The hazard posed by exposure to a chemical via a given route was ranked according to the chemical's European Union risk phrases, and potential for exposure was estimated by the quantity in use and the volatility of liquids or, for solids, potential for airborne particulates.
What are the parameters related to exposure assessment that the user of CB must provide?
1. Acquire complete understanding of the strategy, including R-phrases, quantity of substance in use, and dustiness/volatility of substance.
2. Construct strategy to combine quantity in use, dustiness/volatility, and other determinants, to predict exposure band.
3. Use hazard information with task activities to determine the control guidance level.
4. Select Control Guidance Sheet (CGS)
The COSHH Essentials model is limited to substances under Chemical Hazard Information and Packaging (CHIP) regulations. What substances are excluded from the COSHH model?
Because COSHH Essentials is limited to substances classified under CHIP regulations, the model is not applicable to pesticides, pharmaceuticals, and process-generated hazards such as wood particulate and welding fumes. (Silica dust is also excluded but has been addressed with the HSE development of the silica hazard and task-specific guidance sheets.)
What are the four control approaches (bands) used in the COSHH Essentials model?
Control Approach 1 - General Ventilation (Good standard of general ventilation and good working practices).
Control Approach 2 - Engineering control (Ranging from LEV to ventilated partial enclosure).
Control Approach 3 - Containment (Containment or enclosure, allowing for limited, small scale breaches of containments).
Control Approach 4 - Special (Seek expert advice).
What is control banding?
Approach to controlling exposures when norelevant OELs are available
Control banding focuses resources on
exposure controls
greater potential for harm = greater degree of control
When using control banding, you classify chemical exposures according to
1. Expected toxic outcomes
2. Chemical and physical characteristics
3. Intended process handling
4. Anticipated exposure scenarios
Control banding uses the expected toxic outcomes, chemical and physical characteristics, intended process handling, and anticipated exposure scenarios to
put the chemical agent into a risk class or band which has a control option attached to it
What is the justification for using control banding?
1. Majority of chemical substances do not have published Occupational Exposure Limits (OELs)
2. Employers do not have guidance when selecting controlsfor these chemicals
3. Control options for chemicals with similar toxicity and use conditions tend to be the same
Describe the models used for control banding.
There are several models adopted for control banding-
1. These models have different number of control bandsor levels (minimum four)
2. One of the first models used is the Control of Substances Hazardous to Health (COSHH) Essential developed in England
3. See also models adopted by the pharmaceuticalindustry and nanotechnology posted on Canvas
What does COSHH stand for?
Control of substances hazardous to health
what model for control banding did we use in lab?
COSHH
The COSHH Essentials was developed for
small and medium-sized companies
What are the input variables for the COSHH Essentials model?
1. Toxicity of chemical agent
2. Likelihood of Exposure
• Quantity used
• Ability to become airborne
What is the output for the COSHH Essentials model?
1. Exposure Prediction (paper copy only)• Estimates exposure concentration range (e.g. 5-50ppm)-
2. Control Selection• Engineering controls necessary
Describe the general conceptual model for the COSHH Essential Model
health hazard + exposure potential --> generic risk assessment --> control approach
CB is a valuable tool for
risk management of chemical agents without OELs
Is CB meant to be a substitute for OELs?
no
Does CB eliminate environmental monitoring or IH expertise?
no