cofactors and enzyme inhibition
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
cofactors/ coenzymes
non-protein substances that are bound to enzymes to make them work
cofactors which are inorganic molecules
They work by helping the enzyme and substrate bind together. They don’t directly participate in the reaction so aren’t used up or changed in any way
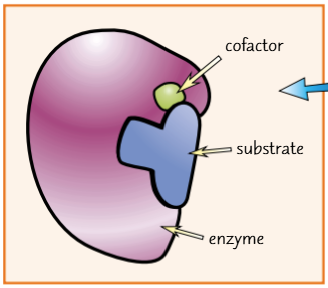
cofactors which are organic molecules
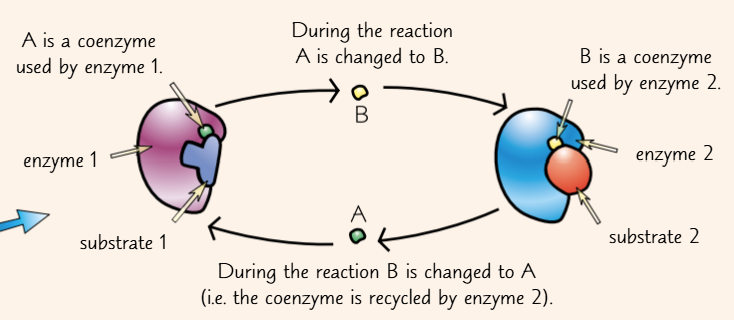
these are called coenzymes
they participate in the reaction and are changed by it
they often act as carriers, moving different chemical groups between different enzymes
they’re continually recycled during this process
vitamins are often sources of coenzymes
competitive inhibition
competitive inhibitor molecules have similar shape to that of the substrate molecules
they compete with the substrate molecules to bind to the active site, but no reaction takes place
instead they block the active site so no substrate molecules can fit in
how much the enzyme is inhibited depends on the relative concentrations of the inhibitor and the substrate
if there’s a high concentration of the inhibitor, it’ll take up almost all of the active sites and hardly any substrate will get to the enzyme
But if there’s a higher concentration of substrate, then the substrate’s chances of getting to an active site before the inhibitor increase. So increasing the concentration of a substrate will increase the rate of reaction
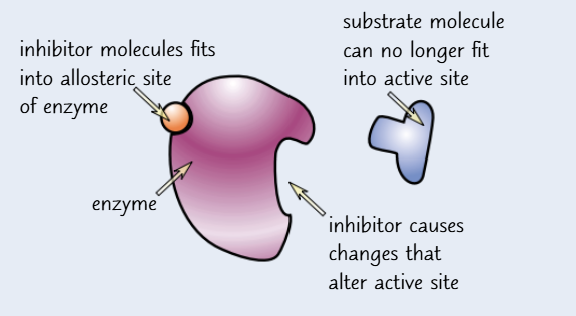
non-competitive inhibation
non-competitive inhibitors molecules bind to the enzyme away from its active site at a site called the allosteric site
this causes the active site to change shape so the substrate molecules can no longer bind to it
they don’t compete with the substrate molecules to bind to the active site because they have a different shape
increasing the concentration of substrate won’t make any difference
inhibitors can be reversable or non-reversable
if they’re strong, covalent bonds, the inhibitor can’t be removed easily and the inhibition is irreversible
if they’re weaker hydrogen bonds or week ionic bonds, the inhibitor can be removed and the inhibition is reversable
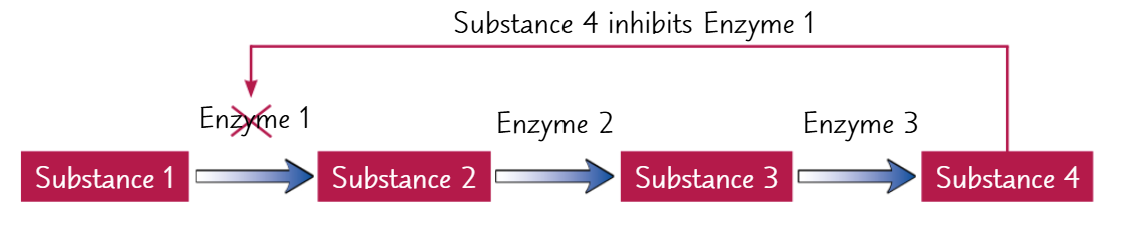
end product inhibition
a metabolic pathway is a series of connected metabolic reactions. The product of the first reaction takes part in the second reaction and so on
each reaction is catalysed by a different enzyme
many enzymes are inhibited by the product of the reaction they catalyse. This is known as product inhibition
end product inhibition is when the final product in a metabolic pathway inhibits an enzyme that acts earlier in the pathway
How are metabolic pathways regulated by end-product inhibition
phosphofructokinase is an enzyme involved in the metabolic pathway that breaks down glucose to make ATP
ATP inhibits the action of phosphofructokinase - so a high level of ATP prevents more ATP from being made
both the product and end-product inhibition are reversable. So when the level of product starts to drop, the level of inhibition will start to fall and the enzyme can start to function again meaning that more product can be made