RS04 - O2 and CO2 Transport
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
What is the total gas in solution equal to?
The sum of dissolved, bound, and chemically modified gas.
Which form of gas in the blood contributes to its partial pressure?
Only dissolved gas.
What is dissolved gas dependent on?
Solubility.
What is bound gas?
Gases bound to proteins, such as hemoglobin.
What is the primary example of a chemically modified gas in the blood?
CO2 converted to bicarbonate by carbonic anhydrase.
What is the PO2 diffusion gradient along the pulmonary capillary?
Arterial end: 40 mmHg
Venous end (alveolus): 104 mmHg
How long does it take for O2 equilibration to occur in a healthy person?
Within 0.25 seconds, or within 0.75 seconds during exercise.
How does a damaged lung affect O2 equilibration time?
It takes longer for equilibration to occur.
What is the effect of decreasing RBC transit time?
It will decrease PO2.
How does O2 diffuse in a healthy lung?
In a healthy lung, O2 diffuses rapidly enough so RBC have plenty of time to take up/saturate with O2.
What is PO2 of interstitial fluid dependent on?
Oxygen consumption and blood flow to tissue.
What happens to tissue PO2 when blood flow is increased?
PO2 increases.
How is oxygen transported through the blood?
Dissolved O2 (2%)
PO2 x solubility of O2
O2 bound to hemoglobin (98%)
What is the oxygen carrying capacity of blood for dissolved oxygen?
2 mL O2 / L blood
Based on a cardiac output of 5 L/min, what is the amount of dissolved oxygen supplied to tissues?
3 × 5 = 15 mL O2/min
What is the resting metabolic O2 demand of tissues?
250 mL O2/min (way higher than the amount of dissolved O2 supplied to tissues)
Can dissolved oxygen meet the demands of tissues?
No, need bound blood to supply the rest of the oxygen to meet metabolic demands.
What is the effect of hemoglobin on O2 carrying capacity?
Hemoglobin increases O2 carrying capacity.
What is the effect of hemoglobin on PO2?
Hemoglobin binds O2 and reduces PO2 in solution.
More O2 does not mean higher PO2; it could be bound to Hb!
What is the maximum amount of O2 that can be bound to hemoglobin per volume of blood?
1 g of Hb can bind 1.34 mL of O2 when 100% saturated.
What is the normal Hb concentration, and what depends on this?
~15 g/100mL. Oxygen carrying capacity of blood is highly dependent on Hb concentration!
What is the total O2 content of blood made up of?
O2 bound to Hb + dissolved O2.
What is the total amount of O2 supplied to tissues by hemoglobin?
1000 mL O2/min. This far exceeds the metabolic demands of tissues!
What is HbA?
Adult hemoglobin; normal hemoglobin that contains 2 alpha and 2 beta chains.
What is methemoglobin?
A form of Hb where iron is in the ferric (Fe3+) state. It cannot bind O2. Erythrocytes contain methemoglobin reductase to convert to regular ferrous state.
What is fetal hemoglobin (HbF)?
A form of hemoglobin in the fetus that has 2 alpha and 2 gamma globin chains. It has a higher affinity for O2 than HbA.
What is sickle hemoglobin (HbS)?
A hemoglobin that has a mutation in the beta chain. DeoxyHb crystallizes into long fibers that deform erythrocytes.
What is glycated hemoglobin?
Hb that has a non-enzymatic addition of sugar residue to the beta chain. This increases with poorly controlled hyperglycemia.
How many O2 molceules can Hb bind?
Four heme groups give Hb the capacity to bind four O2 molecules.
Is binding of O2 to Hb reversible?
Yes, it is reversible because of interactions with globin chains. This is oxygenation (reversible) rather than oxidation (irreversible)
How does the affinity of DeoxyHb change with increasing O2 binding?
DeoxyHb has a relatively low affinity for O2, but the affinity increases with each successive O2 binding event.
What are the two conformations of hemoglobin?
Tensed (T) state: no O2 bound, low affinity for O2
Relaxed (R) state: O2 binding causes conformational change → increased affinity for O2
How much T and R is there at normal O2 levels?
There is usually an equilibrium between T and R states at intermediate PO2 levels.
What is pulse-oximetry?
Pulse oximetry is a non-invasive estimate of the O2 saturation of arterial blood using light passed through the finger.
How does pulse-oximetry work?
Hb in the R state absorbs more blue light and appears red. Light passing through the finger measures the absorbance of pulsing vs non-pulsing light, giving a ratio of oxyhemoglobin and deoxyhemoglobin.
What are the limitations of pulse-oximetry?
Does not measure CO2
Does not account for changes in Hb concentration
Gives a false reading with CO intoxication
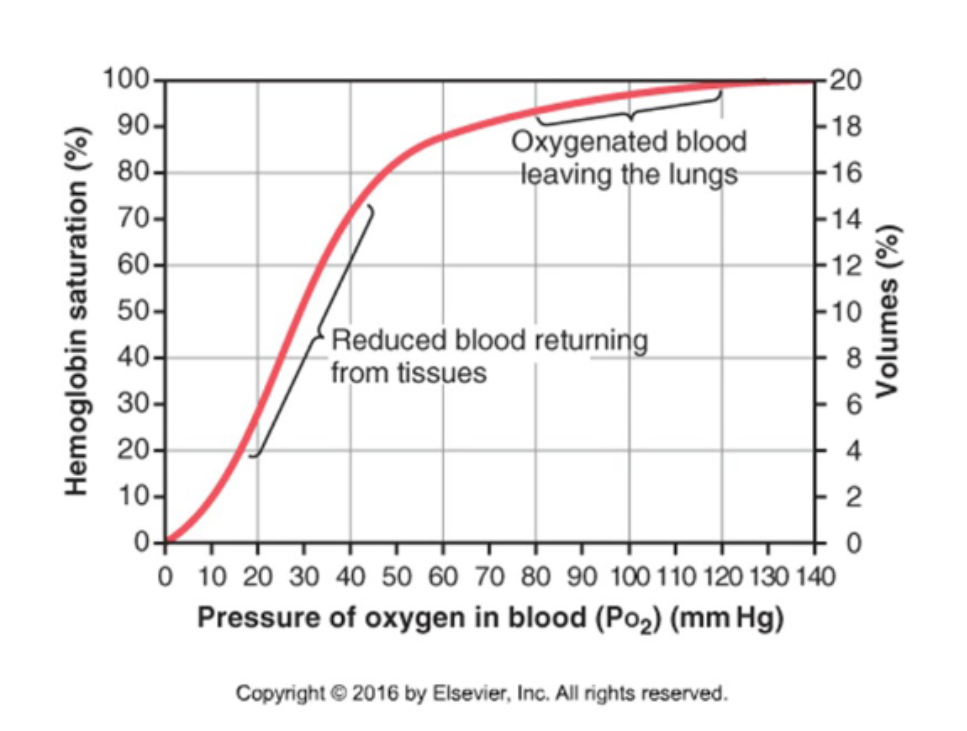
What is the Hb-O2 dissociation curve"?
A graph measuring Hb O2 saturation at various PO2 values.
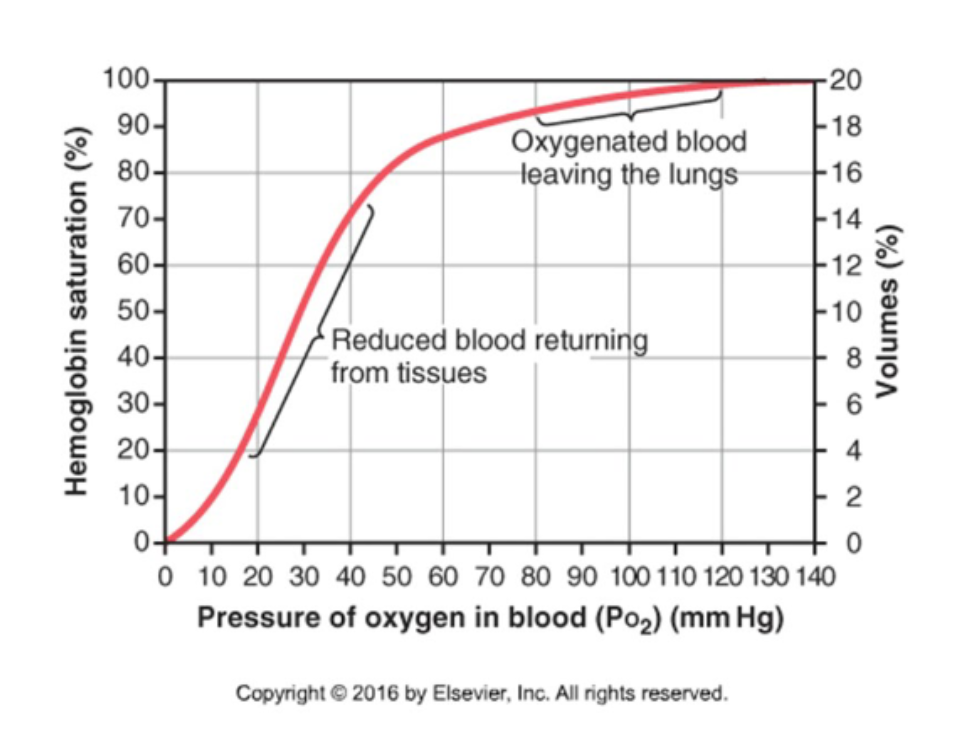
What is the shape of the Hb-O2 dissociation curve?
It is sigmoidal reflecting a change in affinity as more O2 molecules bind. This is called positive cooperation.
How is O2 unloaded from the lungs? (PO2 = 100 mmHg)
Binding of O2 to Hb facilitates diffusion of O2 from alveolar air into plasma.
How is O2 unloaded from peripheral capillaries (PO2 = 40mmHg) into interstitial fluid?
Low PO2 in interstitial fluid facilitates O2 diffusion and removal of O2 from Hb.
What happens to O2 unloading during exercise?
During exercise, PO2 in tissues decreases below 20 mmHg and even more O2 can be unloaded from Hb.
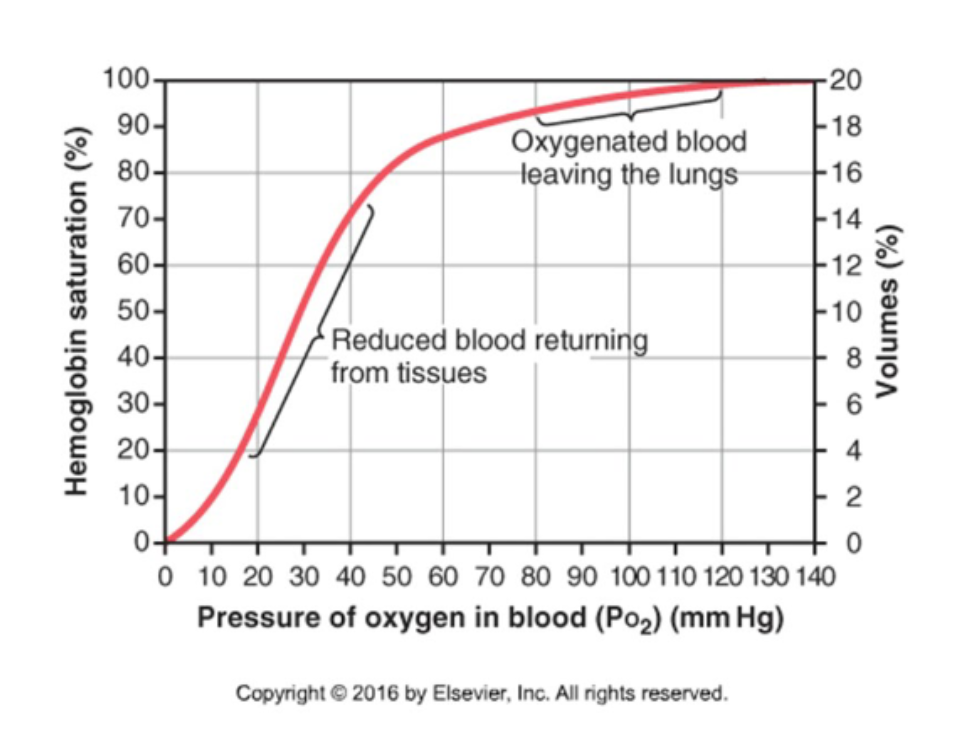
What is the shape of the O2-Hb dissociation curve at tissue and lung PO2 levels?
The curve is steep at tissue PO2 but flat at lung PO2 levels (where blood is fully saturated in Hb).
How is Hb unloading regulated at tissues?
At tissues, O2 consumption regulates O2 unloading. A small difference in tissue PO2 results in a large change in O2 binding to Hb.
How is Hb unloading regulated at the lungs?
In the lungs, atmospheric PO2 has a small effect on O2 saturation. A large difference in atmospheric PO2 results in a small change in O2 binding to Hb.
What does a right shift mean on the dissociation curve?
Increased O2 unloading. Often occurs when metabolic demand is increased.
What does a left shift mean on the dissociation curve?
Increased O2 binding to Hb.
What does increased metabolism cause at peripheral tissues? (Bohr Effect)
Increased metabolism → increased PCO2 → reacts with Hb to form carbamino hemoglobin → decreased pH → increased temperature → decreased affinity for O2 → promotes unloading → right shift
What happens to the lungs during increased metabolism?
Curve is shifted to the LEFT, promoting O2 binding.
How does hemoglobin act as a buffer in plasm?
It binds excess H+, reducing affinity for oxygen. (Accounts for most of the Bohr effect)
What is 2,3-diphosphoglycerate (DPG)?
A product of glycolosis that binds to beta chains of Hb and stabilizes the T (deoxy) state.
When is DPG released?
Tissue hypoxia → increased glycolysis → release DPG.
How does DPG affect Hb affinity for O2?
DPG decreases affinity of Hb for oxygen → increases O2 unloading.
How is O2 binding different in fetal hemoglobin (HbF)?
HbF (2 alpha, 2 gamma) has a higher affinity for O2 than HbA, and it does not bind to DPG so it is insensitive to its effects.
How is the gamma globin related to oxygen affinity?
The y globin is transcriptionally repressed in adults, leading to reduced affinity. One potential target of gene therapy for sickle cell disease would be to turn on transcription of gamma globin.
What factors cause a left shift in the dissociation curve?
Increased pH
Decreased PCO2
Decreased temperature
Decreased DPG
Fetal hemoglobin
(Increased O2 binding)
What factors cause a right shift in the dissociation curve?
Decreased pH
Increased PCO2
Increased temperature
Increased DPG
(Increased O2 unloading)
What does CO2 diffusion at the lungs depend on?
Depends on blood PCO2.
What does CO2 diffusion depend on at the tissues?
Depends on interstitial PCO2.
What are the major forms of CO2 transport in the blood?
Dissolved CO2 (PCO2)
HCO3- (major form of CO2 transport!)
Carbamino compounds
What are the less common forms of CO2 transport in the blood?
Carbonic acid
HCO32- (carbonate)
What is carbonic anhydrase?
Enzyme expressed in erythrocytes that increases the rate of the bicarbonate buffer reaction.
What is the carbonic anhydrase reaction?
CO2 + H2O → H2CO3 → H+ + HCO3-
What determines the direction of the carbonic anhydrase reaction?
It is bidirectional; depends on PCO2
What is the chloride shift?
Excess HCO3- is removed from erythrocytes via the HCO3/Cl exchanger (at tissues). At the lungs, HCO3- is exchanged in the opposite direction as CO2 is unloaded.
What is the haldane effect?
Binding of O2 at the lungs reduces formation of carbamino Hb → decreases CO2 carrying capacity of blood → promotes unloading of CO2.
This is the opposite of the Bohr effect.
What is methemoglobinemia?
Methemoglobin level greater than 1%.
What are the causes of methemoglobinemia?
Congenital: impaired function of methemoglobin reductase
Acquired: drugs with high oxidative capacity (local anesthetics, antibiotics, nitrates)
What are the symptoms of methemoglobinemia?
Tissue hypoxia (cyanosis), shortness of breath, fatigue, exercise intolerance
What is carboxyhemoglobinemia?
Carbon monoxide poisoning; when CO binds to Hb at oxygen binding sites (200x higher affinity)
How is carboxyhemoglobinemia treated?
By administering pure O2.
Co-administration of CO2 will induce hyperventilation
Pure O2 in a hyperbaric chamber (3x the amount in atmosphere)