Comparative Physiology Exam 1
1/151
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
152 Terms
Homeostasis
Regulatory mechanisms thatm aintain internal constancy
Entropy
The 2nd law of thermodynamics states that, over time, differences in temperature, pressure, and chemical potential tend to equilibrate in an isolated physical system
Osmotic pressure refers to what?
The amount of force required to prevent water flow from moving piston
Does compartmentalization only happen on a cellular level?
No, cells can band together to form organs--compartmentalization on a larger scale
Components of cell membranes are able to _________
Move (fluidity is very important)
A cell membrane made up of only __________ fats will be more rigid, while one with a large amount of __________ will be more fluid
Saturated, unsaturated
What are animals?
Multicellular, heterotrophic eukaryotes
No organisms are more ______________ than others: all lineages of organisms on Earth havve evolving for the same amount of time
Advanced
What are the two central questions that comparative physiology seeks to answer?
1) What is the mechanism by which a function is accomplished?
2) How did that mechanism come to be?
What can disrupt internal constancy?
Injury, pathogen invasion, temperature fluctuation, etc.
Regulation
Advantage: permits cells to function in steady conditions
Disadvantage: costs energy
Conformity
Advantage: does not cost energy
Disadvantage: cells are subject to fluctuations in external conditions
Diffusion
The net movement of a substance from a region of high concentration to a region of low concentration
Fick's diffusion equation
J = D (C1-C2/X)
J: mass flux
D: diffusion coefficient
C1-C2: change in cocentration
Diffusion, over ________ distances, is sufficient for biological processes at moving things around
Short
Relative to their environment, cells maintain a net ____________ potential
Negative
Osmosis
Diffusion of water
Water diffuses form areas of _____ solute concentration to areas of ______ solute concentration
Low, high
Cell membranes
How cells keep a barrier between themselves and the outside world
Compartmentalization
Allows cells to regulate internal environment
What are the components of the cell membrane?
-Phospholipids that contain a polar head (hydrophilic)
-Fatty acid tail (non-polar and hydrophobic)
Phospholipid bilayer assembles with the polar phosphate heads facing the _________ and __________ of the cell, with fatty acid chains in the __________
Outside, inside, middle
Species of fish that live in colder water tend to have a higher percentage of hydrocarbon tails that are _____________ while those in warmer temperatures have a smaller percentage.
Unsaturated (increase the demand for fluidity in colder temperatures)
Connections between cell membranes
1) tight junctions
2) separate junctions
3) gap junctions
4) desmosomes
Protein is coded for by _______
DNA
DNA is ________ into RNA which is used as a messenger
transcribed
Every three letters on a strip of RNA is a ________
codon
Primary structure of proteins:
Chain of amino acids in a linear formation
Secondary structure of proteins:
Formation of the protein involving hydrogen bonding
Tertiary structure of proteins:
Beta sheets and alpha helices
Quarternary structure of proteins:
Four proteins come together to function as a whole
Enzymes ______ the necessary energy input required for a reaction to take place
lower
Equation to look at how quickly a substrate is converted into product
V1 = Vmax[Substrate]/(Km + [S])
What is Vmax?
All of the enzymes functioning associated with a substrate
What is Km?
Describes the slope of the reaction velocity curve
Km is determined when the reaction velocity is at ______ of Vmax
Half
Ostracod male bioluminescent mating display equation and the enzyme that drives it
Luciferin + O2 --> Oxyluciferin + light
Driven by luciferase
Three ways enzymes regulate reactions
1) Dial-like regulation
2) Switch-like regulation
3) Allosteric modulation
Channel proteins and the two type
Permit diffusion of solutes or osmosis of water through a membrane--no binding occurs. Two types: aquaporin and gated ion channel
Aquaporins
Water channels in cell membrane. The pore within an aquaporin is so small that it only allows a single waater molecule to pass through at a time
The permeability of a cell membrane to water changes as certain proteins are added to the membrane. Various amino acids are associated with the aquaporin and their job is to:
force water to shift in order to flow through the membrane
Gated ion channels
Only open when certain conditions are met--the necessary environment condition is dependent on the protein
Types of gated ion channels
1) Membrane potential gated: change in external vs. internal charge
2) Stretch/tension gated: opening of protein stretched by the cytoskeleton of the cell
3) Phosphorylation gated: addition of a phosphate group causes the protein to open
4) Ligand gated: the binding of another molecule that forces the protein to change orientation
Transporters and an example
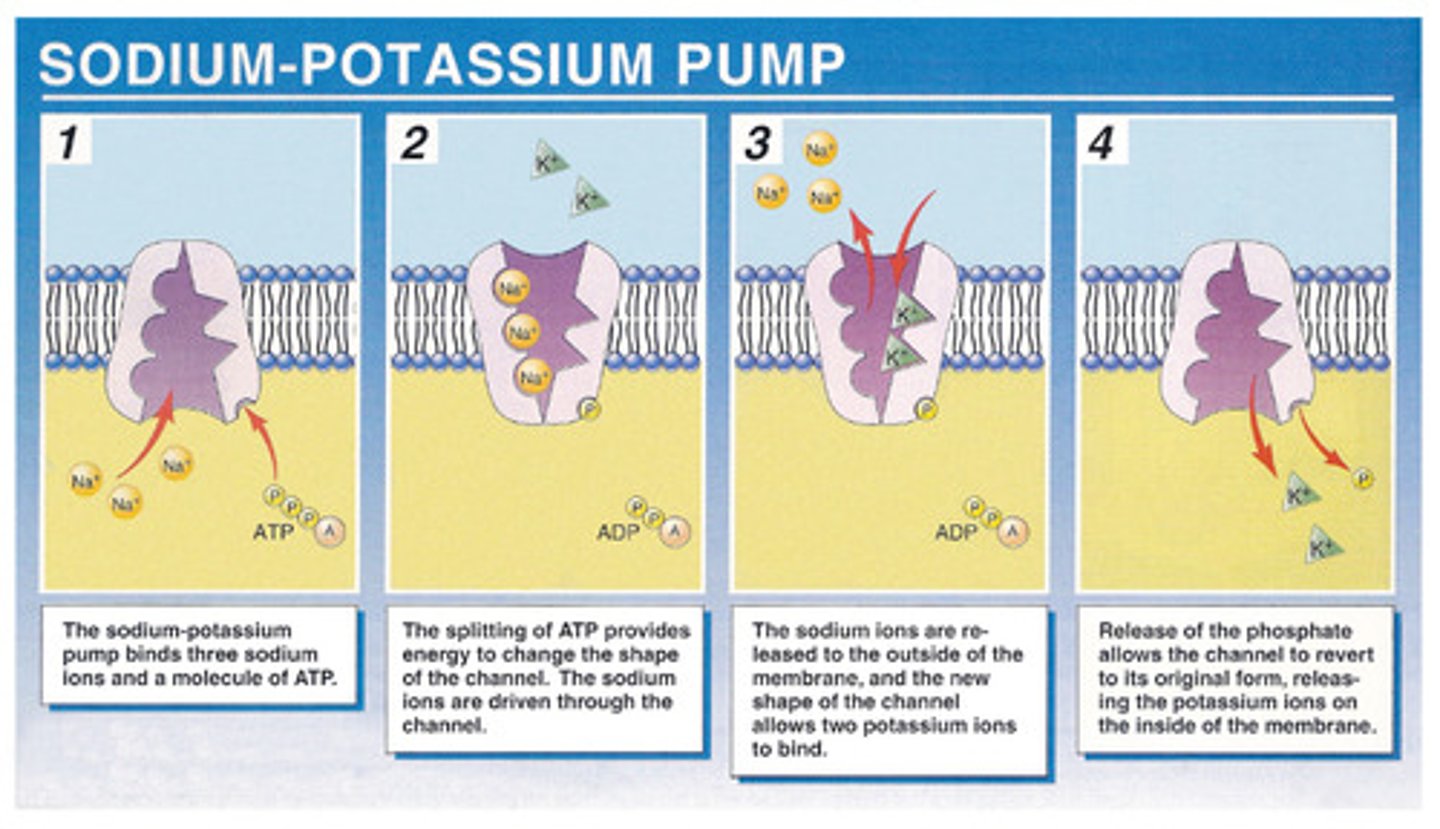
Bind reversibly with certain molecules and move them across membranes intact. Ex: the sodium potassium pump
The sodium potassium pump
For every 3 molecules of sodium pumped out of the cell, 2 potassium molecules are pumped into the cell. This process utilizes ATP--a phosphate molecule is donated to the membrane bound transporter.
Nutrient needs --> ____________ --> ... schematic
Nutrient needs --> feeding --> digestive breakdown --> nutrients delivered
Feeding
The animals evolved feeding apparatus and feeding behavior determines the specific food ingested
Digestive breakdown
breaking down ingested substances by enzyme proteins the animal synthesizes/fermentative breakdown
Nutrients include:
-Proteins
-Lipids
-Carbohydrates
-Vitamins/minerals
How are proteins built?
Through the process of translation by the sequential addition of amino acids in the order specified by mRNA codons that are three bases long. These polypeptide chains then fold to become proteins
What are "essential" amino acids"
Amino acids that must be ingested
How are proteins broken down?
-Amino acids in a chain interact with one another--when the protein is denatured, it causes the protein to unfold due to the loss of these amino acid interactions
How does pepsin function?
There is a "masking sequence" that blocks the active site of pepsin. When the pH gets low, this masking sequence comes off to reveal the active site
Protein digestion involves ___________ and ____________
endopeptidases, exopeptidases
The fate of unused amino acids:
Undergoing deamination --> pyruvic acids, which either go through the Krebs cycle (CO2 + H2O + energy) or gluconeogenesis (glycogen)
Degrading and recycling proteins within the cell
-Process involves ubiquitination
-There are specific enzymes that tag proteins for recycling--helps to ensure the wrong proteins don't get degraded
Lipids
-Most lipid digestion occurs/starts to occur in the pancreas
-Bile salts are an example of amphipathic (polar and non-polar end) molecules--bridges the gap between fat molecules and water molecules
-Lipases break down fats and oils
-Fats require fewer enzymes to break chemical bonds--not fewer in number, but rather fewer in diversity
Absorption
-Happens across cell membranes
-Intestinal villus is present to increase the surface area of the intestines--allows for more space for absorption
Monosaccharides vs. disaccharide absorption
Monosaccharides can be transported and absorbed, disaccharides and polysaccharides must be broken down
The digestion of carbohydrates takes place in both _______ glands and the _____________________
salivary, small intestine
Most prevalent polysaccharide on Earth:
Cellulose
Can animals break down cellulose?
No. If it is present in their diet, they are harboring organisms that are capable of doing it for them.
Enzyme that breaks down cellulose
Cellulase
Energy metabolism
the sum of the processes by which animals acquire energy, channel energy into useful functions, and dissipate energy from their bodies
Entropy
If an isolated system undergoes internal change, the direction of the change is always towards greater disorder (in regards to biology, energy is the capacity to increase order)
High-grade energy
1) Chemical energy: energy liberated or required when atoms are rearranged into new configurations
2) Mechanical energy: the energy of organized motion in which many molecules move int he same direction at the same time. Can be stored or released. We harvest mechanical energy from the environment, but organisms cannot do this
3) Electrical energy: the energy that a system maintains by separating positive and negative electrical charges
Low-grade energy
Heat: the energy of random atomic-molecular motion
Why do different categories of energy matter?
-In animals, chemical energy is totipotent
-Animals cannot use heat to do any form of physiological work
Three major functions that animals use food to perform:
1) Biosynthesis --> chemical energy in exported organic matter
2) Maintenance --> heat
3) Generation of external work --> mechanical energy
Feeding
The energy value of biomass tends to decrease as one moves up trophic levels from produces to consumers
Do closely-related species always have similar diets and feeding structures?
No, they can differ widely
Symbiotic relationships in feeding
Many animals obtain food through symbiotic relationships with microbes
Three categories of microbes with which animals maintain symbioses
1) Heterotrophs--cellulase is an enzyme produced by microbes to break down cellulose
2) Photosynthetic autotrophs--coral polyps that convert sunlight to energy which allows them to create complex carbon chains (coral has symbiotic relationship with zoo-xanthellae)
3) Chemosynthetic autotrophs--bacteria at deep sea vents use the energy generated by the following reaction to grow, reproduce, etc: CO2 + O2 + 4H2S --> (CH2O)x + 4S + 3H2O
Tube worms
An annelid that may be the fastest growing organism on Earth. Has no mouth or gut, relies completely on its symbionts for nutrition
Metabolism
-Anabolism: metabolic processes that build larger molecules from smaller molecules, requires ATP as input
-Catabolism: metabolic processes by which complex chemical compounds are broken down, releases energy
Important things to remember about ATP:
-Cells require ATP to perform work
-ATP is not transported between cells
-Cells do not store ATP in large quantities
The rate at which a cell can do work is dependent on what?
The rate at which it can produce ATP
Anaerobic metabolism
ATP generated in the absence of oxygen, built off anaerobic respiration
Redox reactions
-Reduction: the addition of electrons or hydrogen atoms to a molecule, reducing agents are electron donors
-Oxidation: removal of electrons or hydrogen atoms from a molecule, oxidizing agents are electron acceptors
Glycolysis yield
For each molecule of glucose, you return:
-Two molecules of pyruvic acid
-Two molecules of NAD are reduced to NADH2
-Two molecules of ATP are used
-Four molecules of ATP are formed
Example of anaerobic metabolism (fermentation)
Glucose --> pyruvate --> aspergillus, lactobacillus, or saccharomyces
Phosphagens
High energy phosphate compounds
Aerobic catabolic pathways
-Use oxygen to fully oxidize food molecules to CO2 and H2O
-Capture the energy released by oxidation to make ATP from ADP
What are the four major sets of reactions involved in aerobic catabolic pathways?
1) Glycolysis (occurs in the cytosol)
2) Krebs cycle (occurs in the mitochondria)
3) Electron-transport chain (occurs in the mitochondria)
4) Oxidative phosphorylation (occurs in the mitochondria)
Krebs cycle yields
For each molecule of glucose, you return:
-Six molecules of CO2
-Eight molecules of NAD are reduced to NADH2
-Two molecules of FAD are reduced to FADH2
-Two molecules of ATP are formed
Electron-transport chain
-NAD and FAD were our oxidizing agents --> they get reduced to NADH2 and FADH2
Maximum yield of oxidative phosphorylation is:
25 ATP molecules per molecule of glucose
Total net yield of aerobic catabolism and efficiency:
29 molecules of ATP (31 produced, 2 spent)
Efficiency: 60-70%
Anaerobic vs. aerobic metabolism
1) Aerobic catabolism--steady state, ATP production is slow, but efficient
2) Anaerobic glycolysis--nonsteady state, ATP production if fast, but inefficient
3) Phosphagen use--nonsteady state, ATP production is fast, but inefficient
4) Aerobic catabolism using preexisting oxygen--nonstead state, ATP production is fast, but inefficient
Metabolic rate
The rate at which an animal consumes energy, i.e. the rate at which it converts chemical energy to heat and external work
Why do we care about the metabolic rates of animals?
-Food requirements
-Estimate of total physiological activity
-Ecology (how many organism can an ecosystem support)
How is metabolic rate measured?
As calories per unit of time/watts (J/s)
Food requirements
-A calorie is the amount of heat needed to raise the temperature of 1 gram of water 1 degree Celsius
-The fundamental unit for the measure of energy is the joule (J)--one calorie = 4.186 J
-Humans produce heat at a rate of ~96 W (J/s)
Difference in metabolic rate
Metabolic rate changes over time for any individual organism based on:
-Activity level
-External temperature
-Body size
-Age
-Time of day
-Ingestion of food
Formula used to investigate metabolic scaling
M = aW^b
M: metabolic rate
W: body weight
a & b: constants
How must you manipulate the metabolic scaling formula to study weight-specific metabolic rates?
Divide each side by W:
M/W = aW^(b-1)
Heat
-The energy that matter possesses due to the random motions of the atoms and molecules of which it is composed
-Heat influences the shapes of proteins and therefore influences their functions
Temperature
A measure of the random motion of the atoms and molecules that make up a substance; the more motion, the higher the temperature
Heat moves from _____ temperatures to ______
high, low
What does the transfer of heat do?
Raises the temperature of the object receiving the heat and lowers the temperature of the object losing heat