Structural Isomerism
1/11
Earn XP
Description and Tags
-summaried cardson things needed to know, ques igot wrong bcs i am not confident on idetifiying types of isomers yet
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
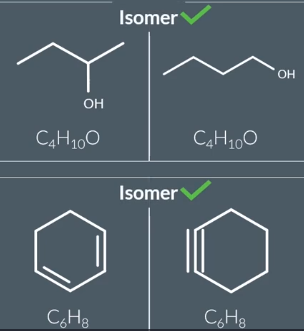
Isomers
same molecular formula, but have a different arrangement of the atoms in space.
same atomic no/ no of e or protons but different no of neutrons
different physical/chemical properties.
picture doesn’t show isomerism as they are same molecular formula but different ways of drawing the molecule→ both called 2-methylpentane

Do these compounds show isomerism?
Yes
These compounds are isomers because they both have the same molecular formula and a different arrangement of atoms.
The molecular formula of both molecules is C6H10. In the first molecule, the triple bond is between the third and fourth carbon of the carbon chain. In the second molecule, the triple bond is between the first and second carbon.

Do these compounds show isomerism?
yes
These compounds are isomers because they both have the same molecular formula and a different arrangement of atoms.
The molecular formula of both molecules is C3H8O
In the first molecule, the functional group is on the third carbon of the carbon chain. In the second molecule, the functional group is between the second and third carbon.

Do these compounds show isomerism?
No
These compounds are not isomers because they don’t have the same molecular formula.
For the first molecule, the molecular formula is C4H10S. For the second molecule, the molecular formula is C4H8S.

Do these compounds show isomerism?
yes
These compounds are isomers because they both have the same molecular formula and a different arrangement of atoms.
The molecular formula of both molecules is C4H8. In the first molecule, the carbons are connected by single bonds. In the second molecule, the third and fourth carbon have a double bond between them.
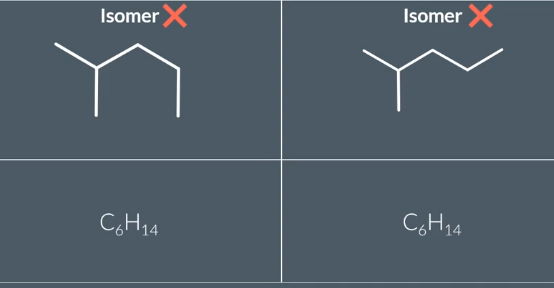
Do these compounds show isomerism?
no
These compounds are not isomers because they both have the same molecular formula (C9H20) and the same arrangement of atoms.
In both molecules, the methyl side chains are on the third and fifth carbon of the longest chain. The molecules are the same molecule, just rotated differently!
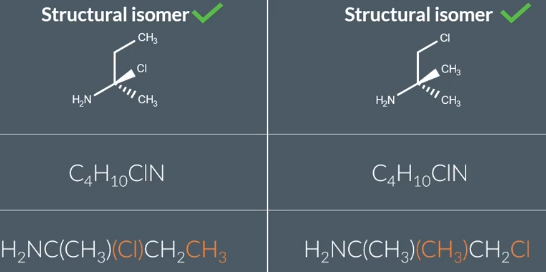
Structural Isomers
same molecular formula, different structural formula
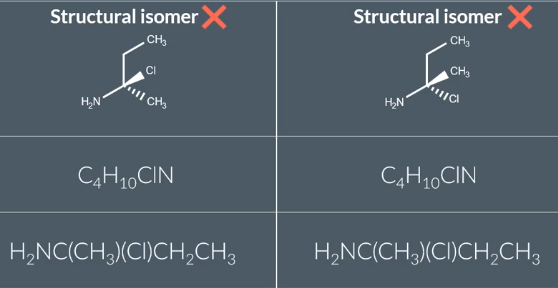
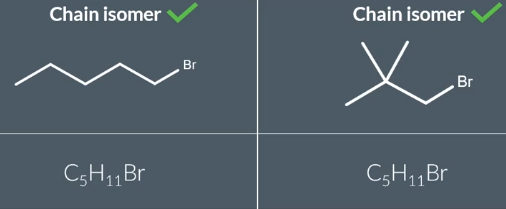
Chain Isomers
when two structural isomers have different carbon chains but the functional group is still in the same position
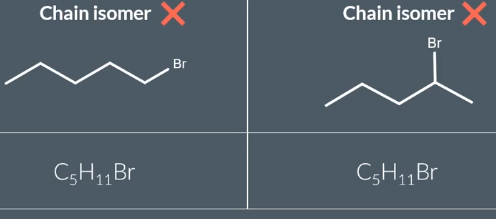
Chain isomer of C4H10
can only draw these not cycloalkanes as C4H10 is an alkane and has the same molecular formula as them
Cycloalkanes do not have same molecular formula
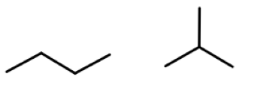
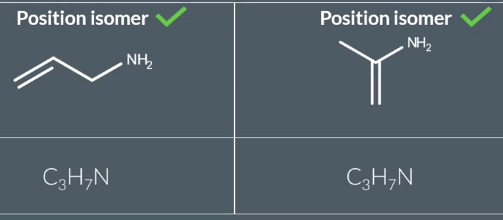
Position Isomerism
2 structural isomers tat have the same type of functional group in a different position in teh compound and same molecular formula
some can be stuctural only not position as seen in the pic
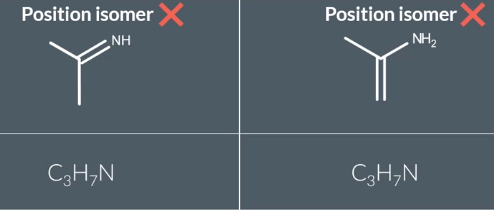
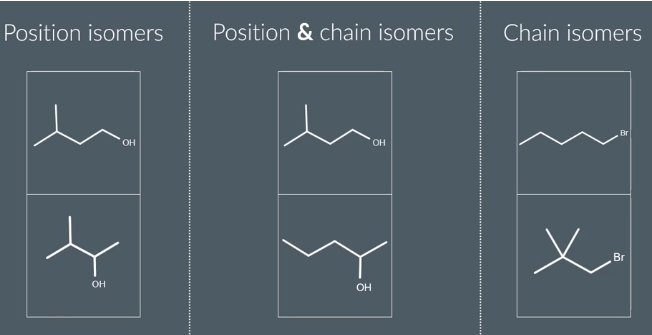
these can co esizt
research w examples
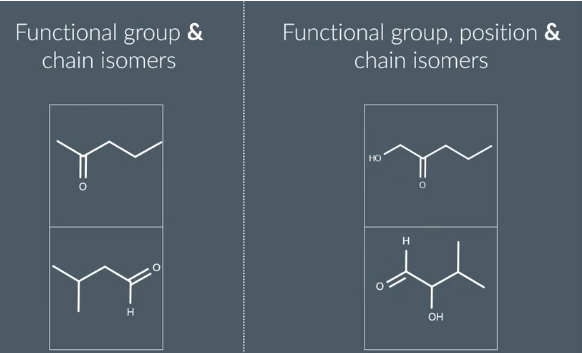

Functional Group Isomers
same molecular formula
different functional group
