Req Practical 2: Carrying out a titration
1/8
Earn XP
Description and Tags
https://www.youtube.com/watch?v=saRBT5oZfh8&list=PL9IouNCPbCxXDlRtCQEG0cGehBvJ7t9Pf&index=11
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What is meant by titration.
Sulfuric acid + Sodium hydroxide → Sodium sulfate + water.
This is a neutralisation reaction.
We have a certain volume of the alkaline sodium hydroxide and we know the concentration.
If we know the volume of the sulfuric acid needed to neutralise the alklai, we can use this to work out the concentration of the acid.
Whats first stage of titration
First we use a pipette to transfer 25 cm³ of sodium hydroxide solution into a conical flask, the conical flask reduces risk of splashing.
We use a pipette filler to draw liquid into the pipette.
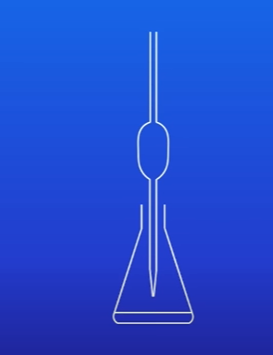
Whats the second step
Add 5 drops of indicator such as methyl orange to the alkali in the conical flask.
Whats third step
Place the conical flask on a white tile so we can see a colour change more clearly.
Whats 4th step
Fill a burette with sulfuric acid
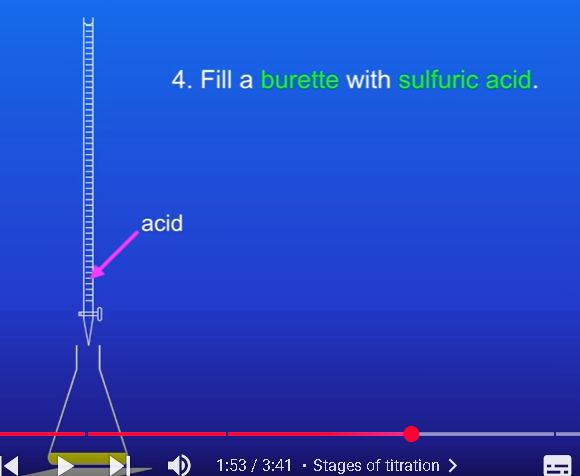
Whats 5th step
Add acid to the alkali until the solution is neutral. We need to add just enough acid for this to happen.
Once we start to see a colour change, we now add the acid drop by drop until the solution is neutral. With methyl orange, the colour will be yellow to red
It is important to swirl the solution to make sure the acid and alkali mix.
Whats 6th step
Read the final volume of acid added from the burette, repeat 3 times and calculate mean.
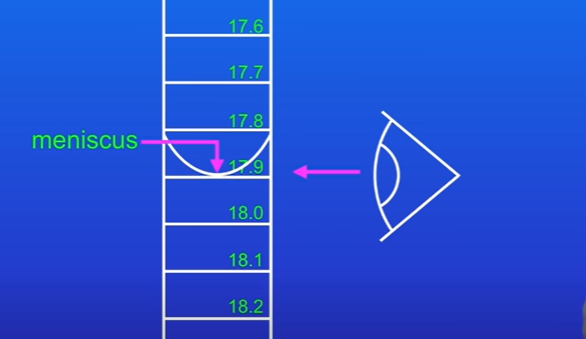
Whats the summary
We know the volume and concentration of the alkali
We know the volume of the acid needed to neutralise the alkali, which means we can calculate concentration of the acid.