19. Amino acid side chains
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
What are the invariant groups of amino acids?
hydrogen
Amino group is a base
Carboxylic acid is an acid
Describe side chains:
20 commonly found in proteins. Either hydrophobic or hydrophilic. If latter, either polar (non charged) or charged ionisable groups. If latter, either positive (basic) or negative (acidic).
List properties of hydrophilic amino acids:
water soluble
Found at the water/protein interface
Provide binding sites for charged molecules and proteins.
List the interactions of amino acids:
Electrostatic, charge and dipoles, hydrogen bonding, CH2 groups cannot hydrogen bond.
lysine (Lys, K) 4 CH2 chain with NH3+ end. PKa 10
Arginine (Arg, R) 3 CH2 chain with multiple NH end. PKa 12
Histidine (His, H) Single CH2 with loop end of CH and NH. PKa 6
What are the charges of these at physiological pH?
Lys (pKa 10) and Arg (pKa 12) are positively charged but His (pKa 6) is a mixture of positive or neutral (important for buffering).
Generally if pH < pKa - 2…. (If the pH is more than 2 units less than the pKa value)…
It is HA (acid) form (so you can ignore the base form)
And if pH > pKa + 2 (If pH is more than 2 units higher than the pKa value)…
It is A- (conjugate base) form and you can pretty much ignore the acid form because there is so little.
If it is somewhere in the middle of this, there is a mixture of the acid and base form you have…
Buffering
There are two negatively charged (acidic) amino acids:
Aspartic acid (aspartate Asp, D) - Single CH2 chain with COO- end
Glutamic acid (Glutamate Glu, E) - 2 CH2 chain with COO- end.
pKa for both is 4.4
What are features of these amino acids?
Fully deprotonated and negatively charged due to low pKa, hydrogen bonding and able to form salt bridges.
There are 4 hydrophilic polar amino acids. What are they?
Asparagine (Asn, N) One CH2 and NH2 end with other stuff
Glutamine (Gln, Q) Two CH2 and NH2 end with other stuff
Serine (Ser, S) One CH2 and OH end
Threonine (Thr, T) Doesn’t have CH2 but CH2O and then CH3 end
They aren’t charged so can’t make salt bridges but have lots of H bonding.
There are two that are hydrophilic or hydrophobic depending on your outlook. What are these?
cysteine (Cys, C) one CH2 and SH end. pKa - 8.3. The SH is not very polar, no H bonding, SH quite reactive
Tyrosine (Tyr, Y) One CH2 and aromatic ring with OH end. pKa - 10. Aromatic ring hydrophobic and bigger than OH. OH can hydrogen bond.
What bonds can cysteines also make? When do they make these bonds?
Disulphide bonds - when they come into close proximity, oxidation can occur and a covalent bond forms between the two SH groups. The entire amino acid is now called a cystine.
Where can you only find molecules with disulphide bonds?
Molecules that have gone through an oxidising environment (only ER in mammals). Anything that comes after this will also therefore have them but the cytoplasm (where most proteins are made won’t as the cytoplasm actively gets rid of any disulphide bonds).
Hydrophobic animal acid properties:
They are found in the centre of globular proteins
Insoluble or poorly soluble in water
Uncharged and non polar side chains
Found in the interior of proteins
At the interface of protein and hydrophobic molecules such as the core of lipid membranes
Anchor proteins or form transmembrane domains.
(Expect to see lots of Cs and Hs)
There are 5 aliphatic hydrophobic amino acids: (in order of increasing length)
Alanine (Ala, A) Just CH3
Valine (Val, V) CH then 2x CH3
Isoleucine (Ile, I) Lots of CH2/3
Leucine (Leu, L) Lots of CH2/3
Methionine (Met, M) two CH2 then S then CH3. - the S is very hydrophobic and very non-polar
There are two aromatic hydrophobic amino acids:
phenylalanine (Phe, F) CH2 then aromatic ring (Like Tyr but OH is missing)
Tryptophan (Trp, W) CH2 then indole ring (double aromatic ring)
There are two special amino acids. What are they and why are they special?
Glycine (Gly, G) Just H. Smallest amino acid, gives flexibility in proteins, not considered either hydrophobic or hydrophilic
Proline (Pro, P) forms a 5 membered ring with Cs and Hs and the NH2+ of the invariant AA. This makes it stiff. Hydrophobic.
What are the different properties you need to know about amino acids?
hydrophobic/hydrophilic
Polar/charged
Positive/negative
Aliphatic/aromatic
Hydrogen bonding (donor or acceptor) - ask beth
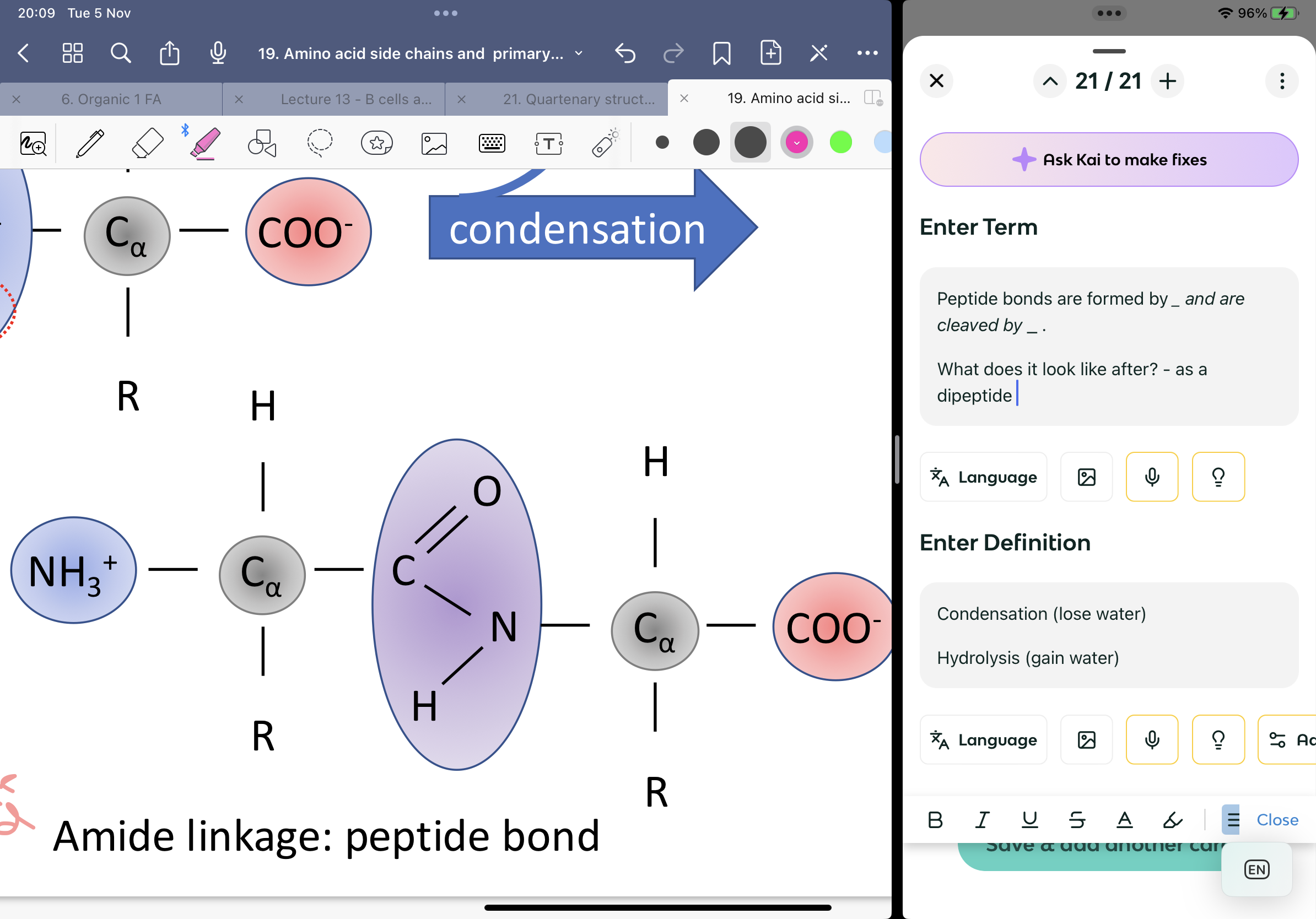
Peptide bonds are formed by _ and are cleaved by _ .
What does it look like after? - as a dipeptide
Condensation (lose water)
Hydrolysis (gain water)
What is the difference between oligopeptides and polypeptides?
oligo - a few AAs (2-50ish)
Poly - many AAs (>50)
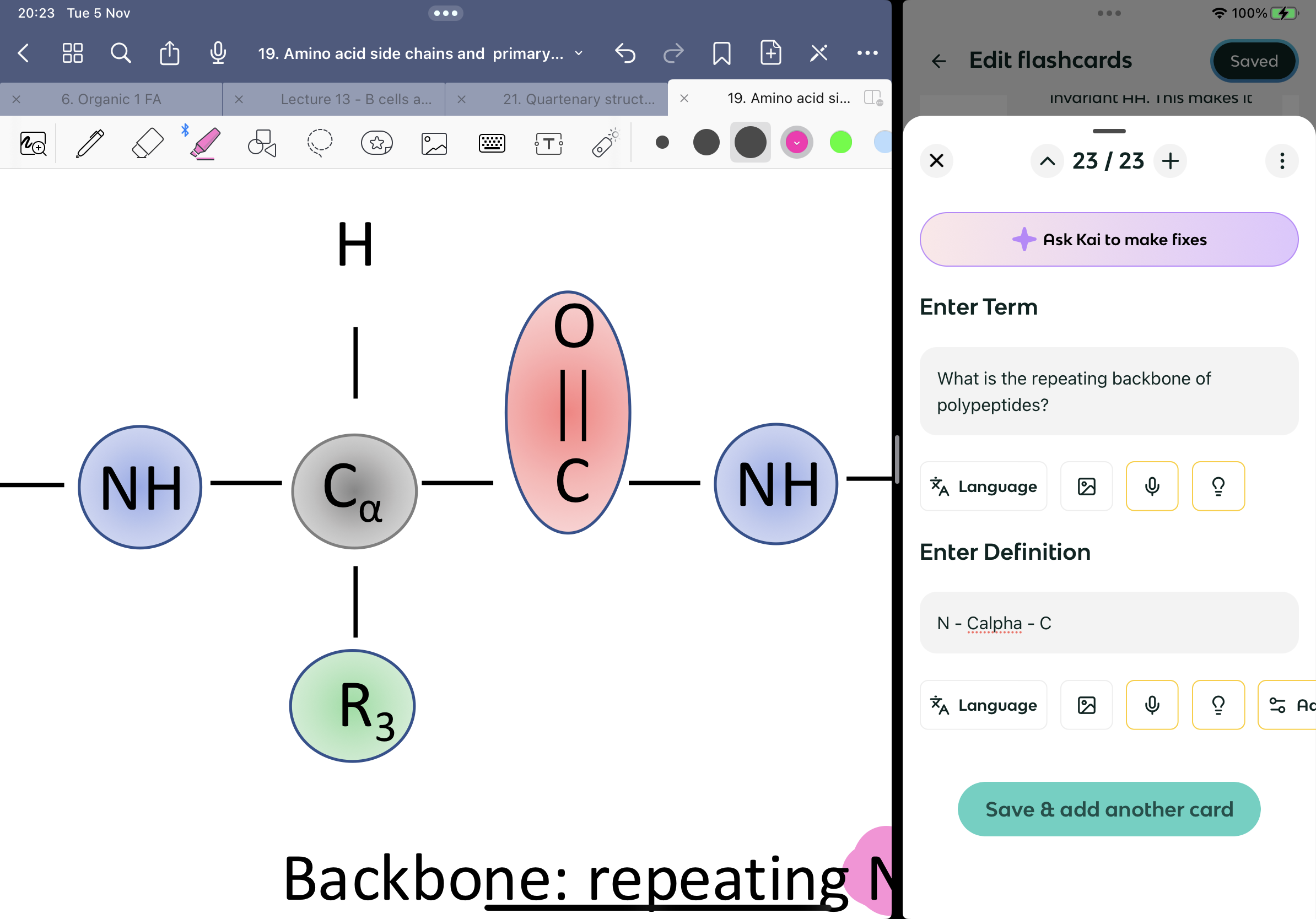
What is the repeating backbone of polypeptides?
N - Calpha - C
What is the definition of a protein?
A polypeptide or complex of polypeptides that has attained a stable three dimensional structure and is biologically active.
How is the naming different for amino acids and chains?
They become amino acid residues and the names go from ending in -ine to ending in -yl. Eg. Proline to prolyl
Because there are 20 AAs, how many possible dipeptides are there?
20×20 = 20² so 400
This means a polypeptide with 100 AAs would be 20^100 long. Only some are made tho - human cells contain 40,000 different proteins (so a lot less than are possible)
What are the biggest and smallest proteins?
Largest - Titin: 27,000 - 33,000 AA
Smallest - Insulin 51AA
Most are 50-2000AA apart from the recent discovery of micro proteins.
The size is often given as molecular mass. What is this measured in?
Daltons (Da) - same mass as a proton. 1g/mol = 1Da
If a protein has a molecular mass of 11kDa, how many g does it weigh?
11kDa = 11,000Da = 11,000g/mol
11,000 × 1.66×10^-24 = 1.8×10^-20
= 0.018 ag
When was the first protein sequenced?
1953 - by Fred Sanger Sequenced insulin. However, now most proteins are sequenced indirectly through sequencing DNA as it is easier.
Why is protein sequencing useful?
protein structure
Protein activity
Protein dysfunction
Protein evolution