Amounts of Substance
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
How to find the volume of gas produced from a known mass of a reactant?
use the mass and Mr to find the moles, then calculate the amount of moles required for the reaction, use the pressure equation
How to make a standard solution?
Record the mass of a weighing boat and solid
Tip the solid into a beaker and record the mass of the emptied weighing boat
Determine the mass of solid used by calculating the difference between the two masses
Dissolve the solid into distilled water
Using a funnel, transfer into a 250cm3 volumetric flask, including washings
Make up to the graduated line by carefully adding deionised water, making sure the meniscus sits on the line
Stopper the flask and invert several times to mix the contents thoroughly
How to find the concentration of a solution of a base?
Fill a burette with a standard solution of the acid
Pipette a known volume of base into a conical flask
Add a few drops of indicator to the base in the flask
Add the solution of the acid from the burette until the indicator just changes colour. This is known as the end point.
Record the volume of acid added: record your burette readings to the nearest 0.05cm3
Perform a rough titration and then sufficient accurate ones until the titres are concordance (within 0.10cm3 of each other)
Calculate the mean titre from the concordance titres
Which values do you use for the mean titre?
the concordant titres (within 0.1cm3 of each other)
How to find the concentration of the base in a titration?
Use the mean titre and the concentration of the acid to find the number of moles
Check the ratio and divide/multiply if needed
Use c = n/v to find the concentration using the known volume of base
How to calculate reacting quantities questions?
Work out the moles of one substance using the given mass and the calculated Mr
Use the balance equation to either multiply or divide depending on the value needed
Use mass = moles x Mr to find the mass of the stated substance
How to find the percentage error
Percentage error = uncertainty / value x 100
(The uncertainty has to be multiplied twice if two measurements are used)
Titration calculations (concentration or volume)
Write the balanced equation
Find out the moles of a substance using the concentration and volume
Apply it to the ratio
Use concentration = n/v to find the concentration or volume
In a titration which substances are placed in the conical flask and burette?
the acid (known concentration) is usually placed in the burette, with the base (known volume in the flask)
How to find the molar mass (Mr) of an acid in titration?
Write the balanced equation
Calculate the amount of moles of the base (in the burette) using the given concentration and volume
Apply this to the balanced equation to determine the amount of moles of acid
Scale up (if needed) to find the number of moles in the total volume
Calculate the Mr using mr = mass / n
How to find the molar mass (Mr) of a base in titration?
Write the balanced equation
Calculate the number of moles of the acid (in the burette) using the given concentration and volume
Apply this to the balanced equation to determine the amount of moles of base
Scale up (if needed) to find the number of moles in the total volume
Calculate the Mr using mr = mass / n
What are back titrations used for and method?
Used to analyse substances that are not soluble in water but do react with acids
Use a known mass of solid reacted with an excess of acid
The resulting solution is titrated with a standard solution of base to determine the amount of acid left
How to calculate back titrations?
Write the balanced equation for the reaction of the solid with excess of acid
Write the balanced equation for the reaction of the base (in burette) with the acid
Calculate the moles of the base using n = v x c
Use the balanced equation ration to determine the moles of acid that react with the base
Scale up if needed
Use the volume and concentration of acid to find the moles of acid at the start
Take away the moles at the start from the moles of acid that react with the base to determine the moles of acid that react with the solid
Use the ratio from the balanced equation to determine the moles of solid
Mass = moles x Mr to find the mass of the solid
What is the water of crystallisation?
When some ionic compounds have water molecules attached to the ions in the crystal. A hydrated salt is a salt which contains water
When hydrated salts are heated, the water is driven off and the compound is said to be anhydrous
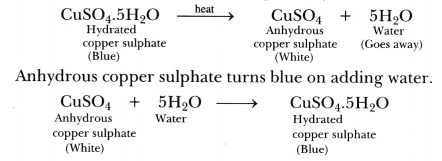
How to calculate the moles of water of crystallisation?
Find out the mass of water (mass of hydrated salt - mass anhydrous salt)
Work out the moles of both the anhydrous salt by calculating the mR
Find out the simplest whole number ratio (divide the biggest moles by the smallest, which becomes 1)
How to calculate the water of crystallisation in a carbonate?
Find the moles of a substance using known values
Use the balanced equation to determine the moles of the carbonate
Scale up if needed to find the total volume
Calculate the mR of the total compound by using mass / moles
Subtract the mR of the hydrated carbonate
Divide by 18 to calculate the number of moles of water
How to calculate percentage yield?
percentage yield = actual yield of product / theoretical yield of product x 100
What is the theoretical yield and how is it calculated?
The number of moles expected in a reaction if the reactants were completely converted into products
This can be found by calculating the number of moles of reactants used and then using the balanced equation to calculate the number of moles of product if the yield was 100%
What is the actual yield and how is it calculated?
The actual number of moles of product formed in the reaction
Determined by calculating the moles of the products using n = m / me
Or by finding a percentage of moles
What are possible reasons for low percentage yield?
Incomplete reaction
Side reactions
Loss of product during transfer or purification
How to prove that a substance is in excess?
Work out the moles of each substance
Use a balanced equation to determine how many moles of one substance is needed (e.g. if the ratio was 1:2, multiply the moles of the 1 substance by two to find out how many it needs)
Show whether the actual is more than the needed, so it is in excess
What is atom economy?
A measure of the efficiency of a reaction in terms of the atoms e.g. if the atom economy is high, there is very little waste, but if it is low there is a lot of waste
Atom economy equation
Molecular mass of desired product (mR) / sum of molecular masses of all products (mR) x 100
How do the types of reaction have an effect on the atom economy?
Addition reactions have an atom economy of 100% (e.g. adding water to a reactant to form one product)
Substitution (swapping elements) and elimination (losing products) have an atom economy of less than 100%
Titration RP- why is it essential that the beakers used for the acid and alkali are clean and dry?
if they are wet they will alter the concentrations of the acid or alkali
What would happen to the concentration of the acid solution if the burette was not rinsed with acid before filling?
Concentration would decrease as water in burette dilutes acid
Why does the conical flask need to be rinsed but not dried before using?
Once the pipette is used to transfer a known amount (moles) of solution, adding water would not affect the number of moles
Is it essential to clean and dry the beaker and volumetric flask before using them to make a standard solution?
No- as the solution will be made up in deionised water the beaker and flask need to be clean but not dry
Why is it essential to include the washings when making up your solution?
The traces of solution coating the beaker + stirring rod will contain some of the solid so must be transferred to the flask
How would the calculated value for the concentration differ from the actual concentration if washings were not included?
Less solute would be transferred (less moles) so calculated concentration would be higher as it assumes all mass is transferred
Relative atomic mass definition (Ar)
The weighted average mass of all the isotopes relative to 1/12th the mass of an atom of carbon-12
What is the relative atomic mass on the periodic table?
The atomic mass (top) number- it is the weighted average of all the isotopes
How to calculate the relative atomic mass- involving isotopes?
(Mass x abundance) + (mass x abundance) / total abundance
Relative molecular mass definition (Mr)
The mass of a molecule of the compound relative to 1/12th the mass of an atom of carbon-12
How can the relative molecular mass and relative formula mass be found? (Mr)
Adding together the relative atomic masses of all the atoms in the molecule or the formula
Relative formula mass definition
The mass of one formula unit of an ionic compound relative to 1/12th of the mass of an atom of carbon-12
Mole definition
The amount of substance that contains as many particles as there are in exactly 12g of carbon-12 (also called the Avogadro Constant)
What is the molar mass?
The mass of one mole of substance is its Mr in grams
Equation for moles in a solid
Mass (g) = molar mass (Mr) x moles (mol)
Equation for moles in a solution (dissolved in water)
Moles (mol) = vol (dm3) x conc (moldm-3)
Equation for mass in liquid (can be used to calculate moles)
Mass (g) = density (gcm-3) x vol (cm3)
Mg g kg tonnes conversions
Mg x 10-3 = g g x 10-3 = kg kg x 10-3 = tonnes
Tonnes x 103 = kg. kg x 103 = g g x 103 = mg
Equation for the number of particles/atoms in a given mass
number of particles = number of moles x Avogadro’s constant (6.02 × 1023)
(calculate moles first using n = m/Mr)
Cm3 dm3 m3 conversions
NOTE concentration for moles is in dm3
Cm3 × 10-3 = dm3 dm3 × 10-3 = m3
M3 × 103 = dm3 dm3 × 103 = cm3
What is the ideal gas equation?
pV = nRT
p = pressure in Pa
v = volume in m3
n = number of moles
R = gas constant (given in Q)
T = temperature (C + 273 = K)
Density equation
mass (g) = density x volume (cm3)
Empirical formula definition
The simplest whole number ratio of atoms of each element present in a compound
How to calculate empirical formula
Convert mass to mole (find the moles by using Mr and percentage mass if given)
Divide the biggest number by the smallest, so the smallest is 1
Express as a ratio and multiply till whole
Common fractions for empirical formula: 0.25, 0.5, 0.,33, 0.75, 0.67
0.25 = x4
0.5 = x2
0.33 = x3
0.75 = x4
0.67 ×3
Molecular formula definition
The actual number of atoms of each element present in a molecule
How to calculate molecular formula
Calculate empirical formula
Calculate relative mass of the empirical formula (if H20, = 2+16)
Use the Mr (given in Q) to calculate how many times bigger the molecular formula is (give it by relative mass) compared to empirical formula