Midterm 2
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
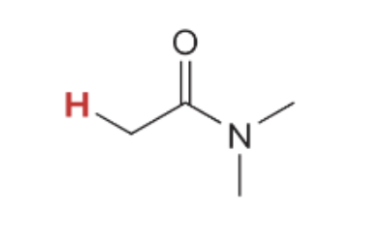
pka value for
30
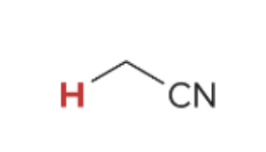
pka value for
25
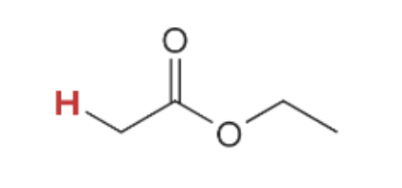
pka value for
25
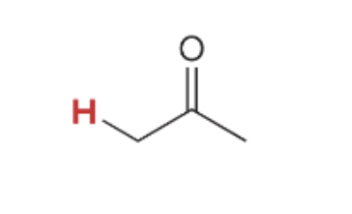
pka value for
19.2
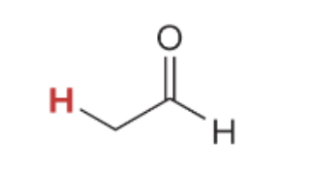
pka value for
17
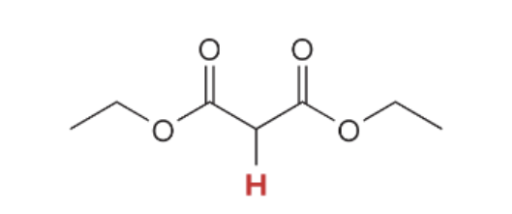
pka value for 1,3 Diester
13.3
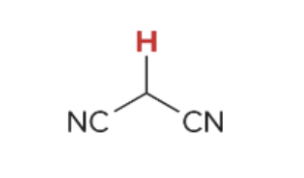
pka value for 1,3 Dinitrile
11
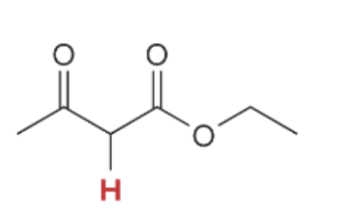
pka value for Beta Keto Ester
10.7
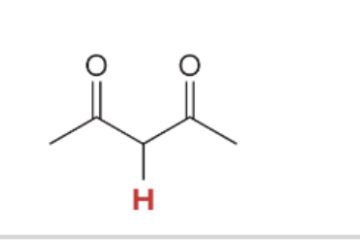
pka value for Diketone
9
formation of an enol
enolate with ketone
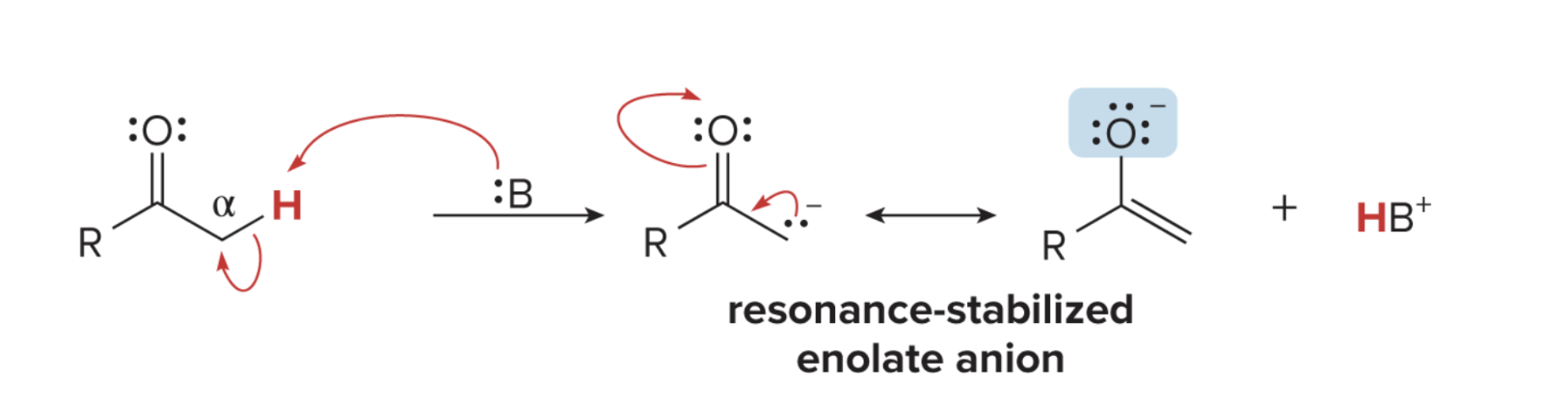
Enolate Formation (basic, NaOH or LDA)
alpha proton gets deprotonated
base with a lone pair takes the proton + the electron forms double bond on the alpha carbon
carboxyl deprotonates and forms alkoxide
enolate is formed + HB^+
enolate with alkyl halide
enolate with aldehydes
Acetoacetic Acid Synthesis
Malonic Ester Synthesis
Aldol Reaction w/ heat
Claisen Reaction
Michael Reaction
Robinson Annulation
Halogenation in Acid
Halogenation in Base
Halogenation
crossed aldol
isoelectric point
aldol reaction without heat (NaOH, NaOMe, NaOEt, KOH)
On main carbon:
Alpha carbon deprotonated, double bond forms
C=O, broken, alkoxide formed
alpha carbon w/ dbl bond attacks carbonyl of another carbon
Alkoxide —> C=O again
On other carbon:
C—C double bond created
C=O bond —> C—O^-
O gets protonated

Amino Acid
glycine
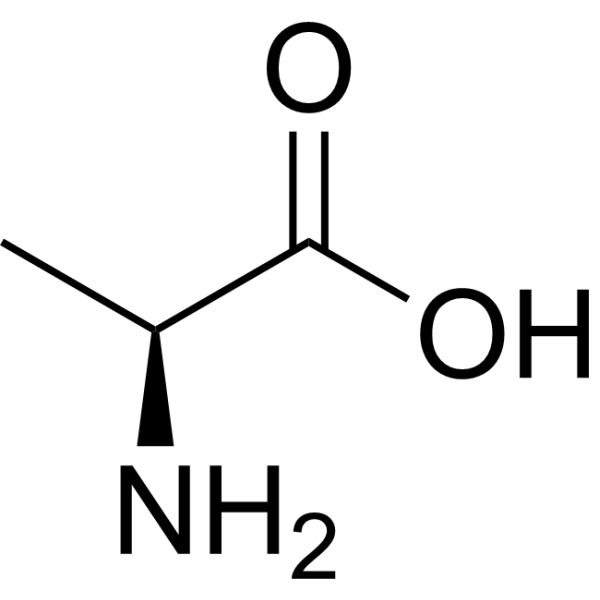
Amino Acid
alanine
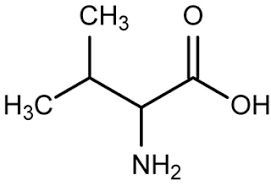
Amino Acid
Valine

amino acid
phenylalanine
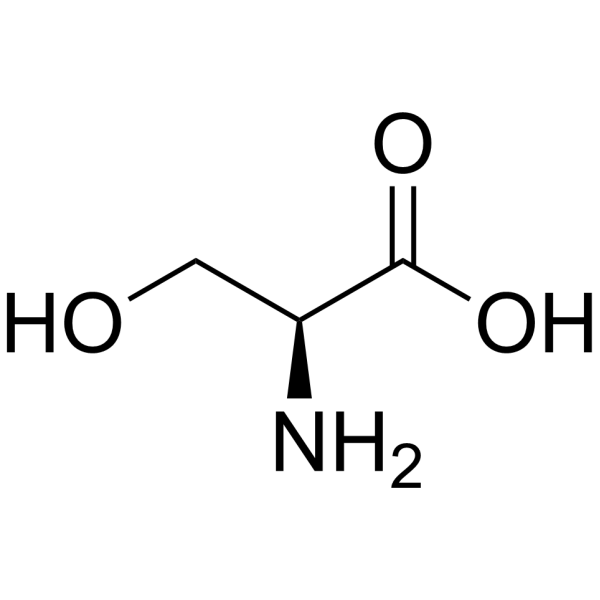
Amino Acid
serine
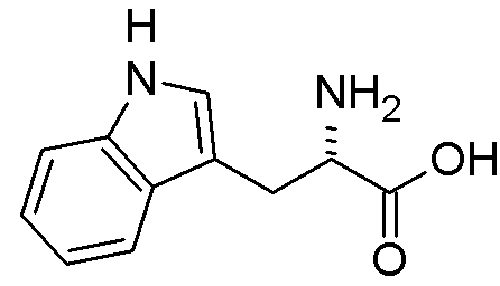
Amino Acid
tryptophan
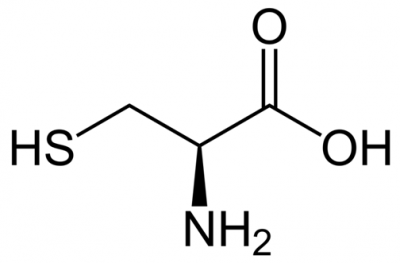
Amino Acid
cysteine
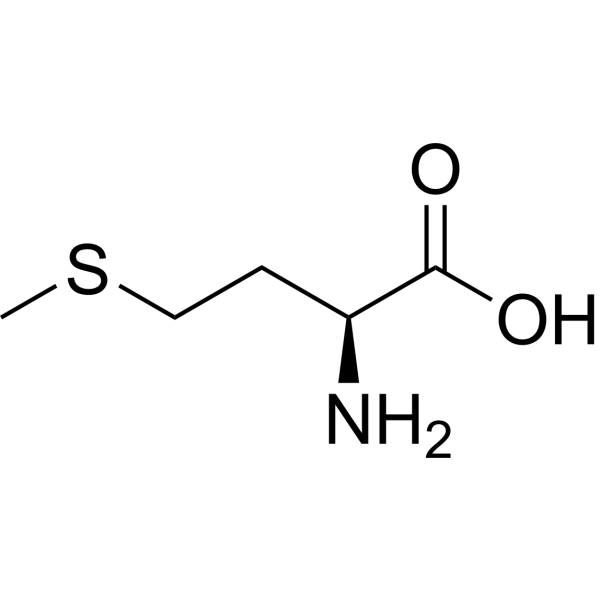
Amino Acid
methionine
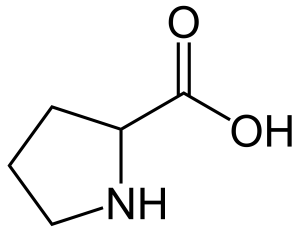
Amino Acid
proline

Amino Acid
tyrosine