3.9 Separation of Solutions and Mixtures Chromatography
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
Chromatography provides a method of:
Separating a mixture of solutions
Chromatography separates a mixture of solutions based upon:
Polarity differences
Polarity differences between solutions are sometimes considered:
Solubility
Types of chromatography:
Paper, Thin layer, Column
Most common type of chromatography in AP Chemistry:
Paper
Methodology for paper chromatography:
A line is drawn (typically in pencil) near the end of the strip of paper. A drop of the sample to be separated is placed on that line. The paper is placed in an (ideally) sealed container with a shallow layer of solvent where the bottom of the paper is touching the solvent, but the line is not. Time passes and the solvent and dyes will separate. Remove the paper before the solvent reaches the top of the paper. Measure the height of the leading edge of the solvent and measure the leading edge of each dye.
Diagram showing methodology for paper chromatography:
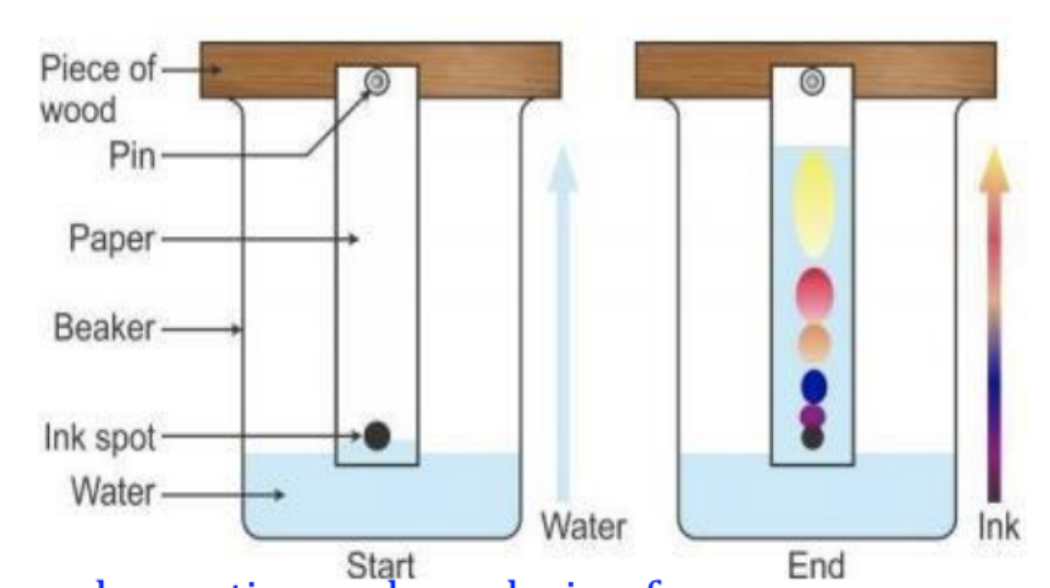
Rf=
Distance travelled by dye / Distance travelled by solvent
Rf stands for:
Retention factor
Rf is same/unique for all solutes
Unique
d ink (subscripted) =
Farthest distance the dye travelled
d ink (solvent) =
Farthest distance the solvent travelled
In paper chromatography, the same compound will / will not move at the same rate relative to ______ on different trials.
Will, The same solvent
In paper chromatography, does Rf factor always differ between compounds?
Yes
Why does Rf factor always differ between compounds in paper chromatography?
Different compounds will have an at least slightly different polarity to other compounds
In paper chromatography, the more similar in polarity the sample is to the solvent the farther / less farther it will travel
Farther
In paper chromatography, polar water will have polar samples travel far / a short distance and nonpolar samples travel far / a short distance.
Far, a short distance
In paper chromatography, nonpolar benzene will have polar samples travel far / a short distance, and nonpolar sample travel far / a short distance.
A short distance, far
How to identify a sample using chromatography?
By comparing Rf values
Is identification of a sample using chromatography possible by comparing distances?
No
The stationary phase is the:
Paper
The mobile phase is the:
Solvent
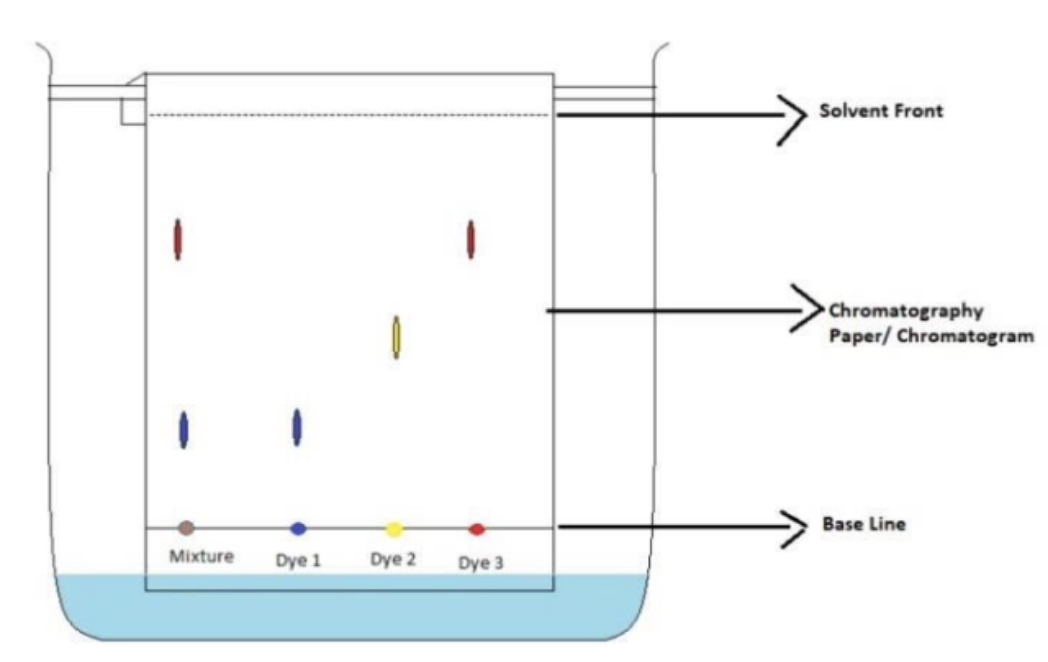
In the image, does the mixture contain dye 2?
No
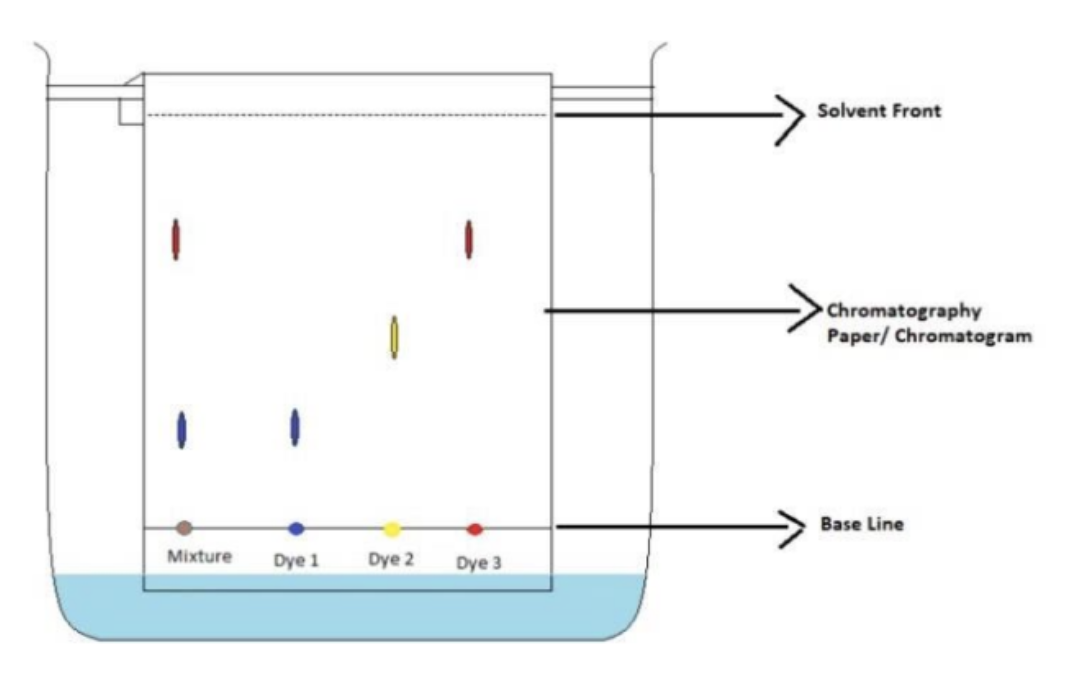
In the image, how do you know that the mixture does not contain dye 2?
There is not a yellow dot in the mixture column
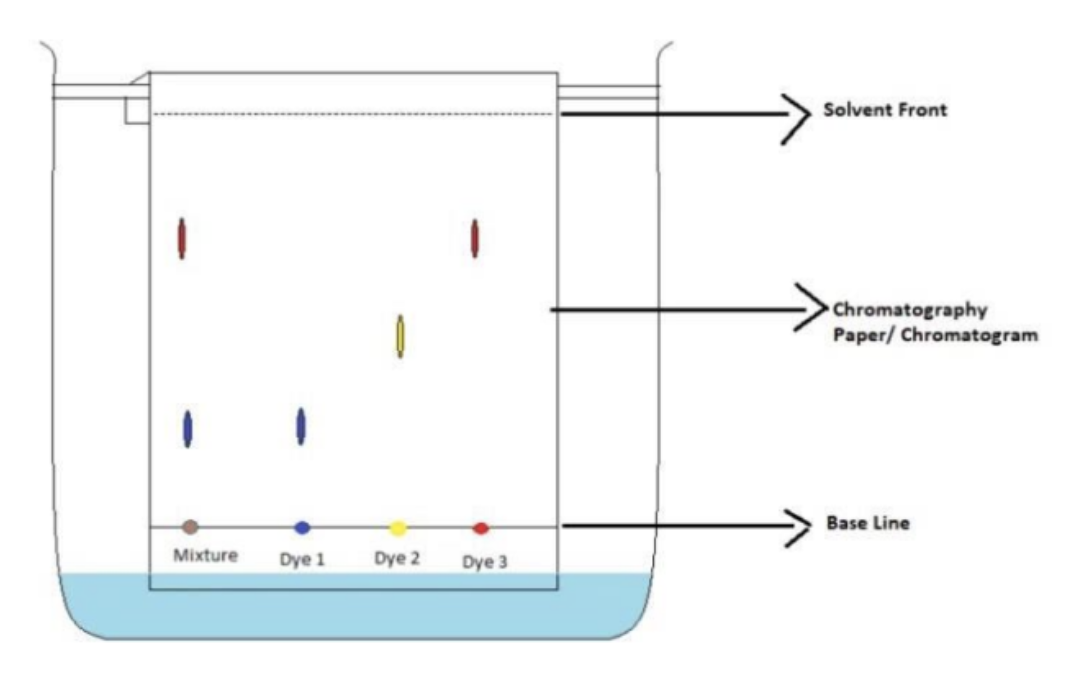
In the image, the mixture contains:
Dye 1 and 3
Is it uncommon for dyes to have multiple parts which would look like multiple dots different distances from the base line
No
Is thin layer chromatography similar to paper chromatography?
Yes
How is thin layer chromatography different from paper chromatography?
Separation occurs on a layer that is on top of a thin sheet of plastic
Examples of layers used in thin layer chromatography:
Silica or alumina
Are layers used in thin layer chromatography polar or nonpolar?
Polar
Are layers used in thin layer chromatography always polar?
No
Thin layer chromatography can be used to separate:
Some samples that are not colored to the naked eye
How to see separated samples not colored to the naked eye when performing layer chromatography?
By using UV light
How does UV light help to see separated samples not colored to the naked eye when performing layer chromatography?
It helps to fluoresce solvent to see substances
When using UV light to see separated samples not colored to the naked eye when performing layer chromatography, the dark spots represent:
Location of components of the mixture
Thin layer chromatography is frequently used with:
Amino acids
In thin layer chromatography, the same compound will move at the same / a different rate relative to the _____ on different trials.
Same, solvent
Is Rf factor same for different compounds in thin layer chromatography?
No
Why is Rf factor not the same for different compounds in thin layer chromatography?
Different compounds will have at least slightly different polarities
In thin layer chromatography, typically, the more nonpolar the sample is, the more/less farther it will travel.
More
In thin layer chromatography, typically, the more polar the sample is, the more/less farther it will travel.
Less
Must leading edge be identified in thin layer chromatography?
Yes
Must leading edge be allowed to reach the top in thin layer chromatography?
No
In thin layer chromatography, identification of a sample is possible by:
Comparing Rf values
In thin layer chromatography, identification of a sample is not possible by:
Comparing distances
Job of column chromatography:
Separate a mixture in solution
Methodology for column chromatography:
Steel wool is placed at the bottom of a burette to prevent the gel from escaping. A burette is filled usually with very polar silica or alumina gel. The mixture that is to be separated is placed at the top of the burette and is flushed time and again with nonpolar solvent (usually). (pour solvent, wait and watch, repeat). The mixture separates and each phase is collected in a beaker or flask one at a time.
Diagram of column chromatography:
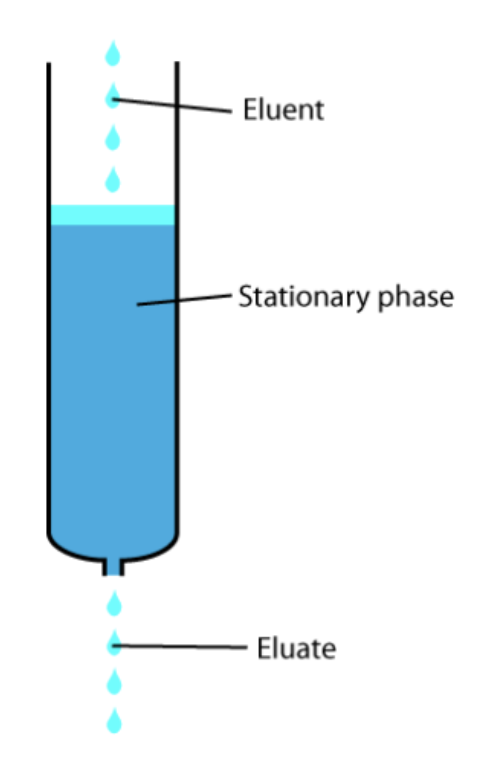
In column chromatography, usually, the most polar parts of the mixture will travel the fastest/slowest and the least polar will travel the fastest/slowest
Slowest, fastest
What should you do after separating one part in column chromatography?
Speed up the movement of the remaining part(s)
How to speed up the movement of the remaining part(s) after separating one part in column chromatography?
By using a new solvent
In column chromatography, using a new solvent to speed up the separation of parts is for:
Separation, not analysis
How to identify the component that you separated in column chromatography for which you used a new solvent to speed up the process?
By using a different method of chromatography
Distillation is a method of:
Physically separating mixtures
Distillation separates substance based on:
Differences in boiling point and intermolecular forces
Process of distillation with diagram:
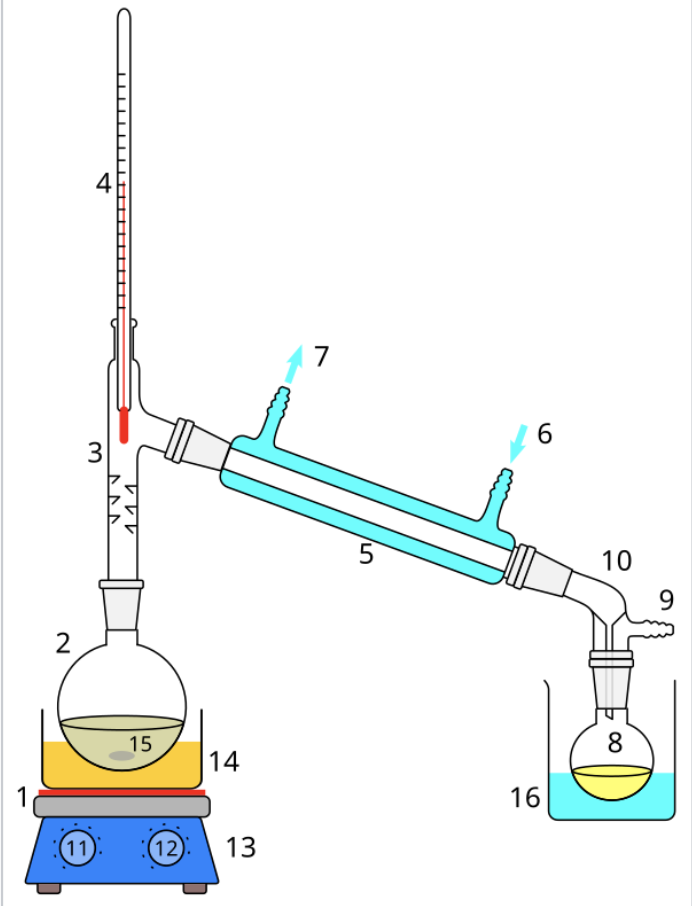
The starting liquid 15 in the boiling flask 2 is heated by a combined hotplate and magnetic stirrer 13 via a silicone oil bath (orange, 14). The vapor flows through a short Vigreux column 3, then through a Liebig condenser 5, is cooled by water (blue) that circulates through ports 6 and 7. The condensed liquid drips into the receiving flask 8, sitting in a cooling bath (blue, 16). The adapter 10 has a connection 9 that may be fitted to a vacuum pump. The components are connected by ground glass joints (gray).
Techniques for separating mixtures:
Chromatography (paper, thin-layer, column) and distillation
Applications of distillation include:
Distillation of fermented beverages, Desalination of salt water, Separation of crude oil into fuels and other petroleum products
The separation of crude oil into fuels and other petroleum products often occurs through:
Fractional distillation
Typically, if the boiling points are close together, simple/fractional distillation is a better technique to separate substances
Fractional
Fractional distillation leads to:
Better purification
Why does fractional distillation lead to better purification?
Allows the vapor to condense and revaporize several times
With all types of chromatography, you will need to recognize:
Which part of the system will interact with the materials being tested
With all types of chromatography, you have to consider:
Intermolecular forces for all components