Ch2 Adaptive Quiz: Water, the solvent of life
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Which of these atoms are electronegative and participate in hydrogen bonding?
oxygen and nitrogen
Which molecule is a hydronium ion?
H3O+
What medical condition does NOT result in acidosis?
hyperventilation
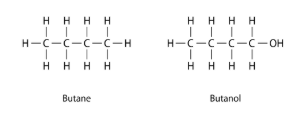
Why does butane have a boiling point of only -0.5.C, whereas butanol has a relatively high boiling point of 177.C?
Butanol’s electronegative oxygen group participates in intermolecular hydrogen bonding
Which of these solutions has the highest OH- concentration?
baking soda solution (pH=9)
Generally, physiological pH:
is near 7
Which molecule is NOT a water soluble?
lipid
What is the pH of a 0.010M solution of sodium hydroxide?
12
What does the Henderson-Hasselbalch equation allow you to calculate?
either the pH, pKa, or molar ratio of the proton donor and acceptor, given the other two variables
How many hydrogen bonds can form between guanine and cytosine?
three
Which statement is false for the ion product of water at 25C?
Kw= neutral pH
Why is the phosphate buffer system particularly effective for biological tissues?
Which statement is false regarding the properties of water?
the molecular geometry of the hydrogens bound to oxygens is triangular
What is the pH of a 0.150 M solution of acetic acid (pKa =4.75) at 25C?
2.8
What is the [OH-] in a 0.010M sulfuric acid (H2SO4) aq solution at 25.C?
5.0 × 10-13 M
Which statement is false for the Henderson-Hasselbalch equation?
the equation describes the titration curves for all acids.
Why is the bicarbonate buffer system more complicated than the other buffer systems?
buffering depends on the concentrations of H2CO3, HCO3-, aq CO2, and gaseous CO2
Which statement if false regarding osmolarity?
A 1 mM NaCl solution has the same osmolarity as a 1mM glucose solution
What is the pH of a solution with an H+ concentration of 10-9 M?
9
Calculate the osmotic pressure of a solution at 300K that is 0.500 M in sucrose and 0.500 M in KCl. (Note: 1 J/L = 1000 Pa = 1kPa)
3750 kPa