chemistry help me
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
HCl (aq) + NH3 (aq) —> NH4+ (aq) + Cl- (aq)
The chemical reaction between HCl (aq) and NH3 (aq) is represented above. A student combined equimolar amounts of HCl (aq) and NH3 (aq), both solutions initially at 24 degrees celsius, in a coffee-cup calorimeter. The students observes that the mixture reaches a temperature of 28 degrees celsius. Based on the experimental results, which of the following can be concluded about the reaction?
It is an exothermic process, because energy is released by the reaction and is gained by the reaction mixture.
When pellets of NaOH (s) are added to a flask of water, it is observed that the temperature of the water increases as the pellets dissolve. Which of the following claims about the observed dissolution of NaOH (s) in water is most accurate?
It is an exothermic process because heat energy is absorbed by the water as the NaOH (s) dissolves in it.
A student carefully drops a 9.0g solid Zn pellet initially at 50.0 Celsius degrees into an insulated cup containing 30.0 g of water at 27.8 degrees Celsius. The student predicts that the temperature of the water will increase after the pellet is added. Which of the following statements is the best justification for the student’s prediction?
Collisions between the water molecules and the surface of the Zn pellet will result in the transfer of energy, increasing the average kinetic energy of the water molecules.
Two aqueous NaCl solutions of equal volume and concentration were kept in flasks and held at different temperatures. The two solutions were combined in a larger flask. Based on this information, which of the following predictions is correct?
The average kinetic energy of the particles in the cooler solution will increase as they collide with the particles from the warmer solution.

The table provides data for two CH3OH (l) samples. Based on this information, which of the following statements describes what happens when these samples are initially mixed, and why?
The CH3OH molecules from sample 1 transfer thermal energy to the CH3OH molecules from sample 2 through collisions because the average kinetic energy of the molecules in sample 1 is greater.
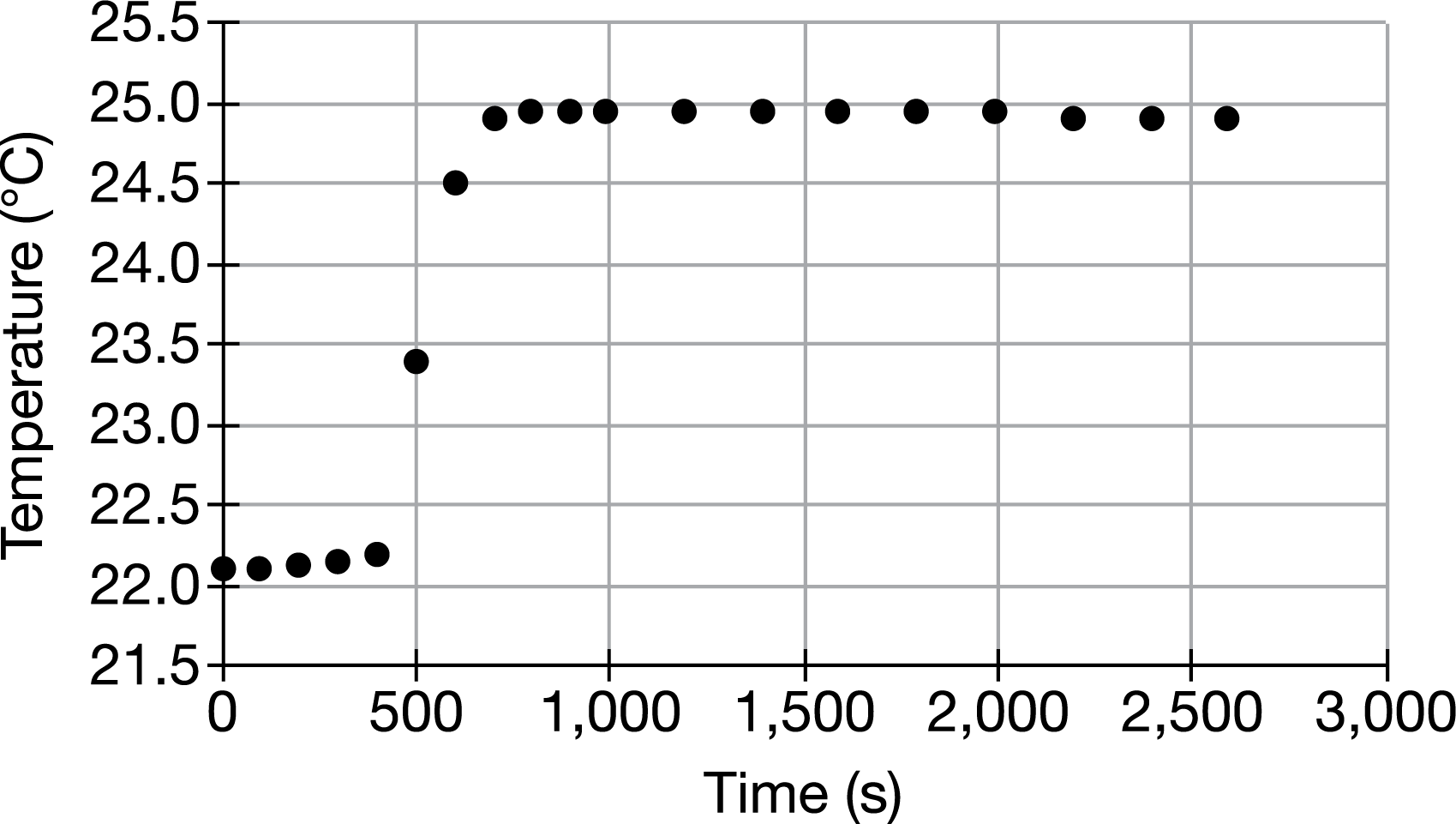
The graph above shows the changes in temperature recorded for the 2.00 L of H2O surrounding a constant-volume container in which a 1.00g sample of benzoic acid was combusted. Assume that heat was not absorbed by the container or lost to the surroundings, that the density of H2O is 1.00g/mL, and that the specific heat capacity of H2O is about 4.2 J/g(C). Based on this information, estimate how much heat was released from the combustion of the benzoic acid sample.
0.25kJ