Histopath
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
90 Terms

ID the pathology at the tip of the pointer
Atrophy

ID what’s at the tip of the pointer
Cholesterol clefts

ID what’s at the tip of the pointer
Metaplasia

ID what’s at the tip of the pointer
Hemosiderin

ID what’s at the tip of the pointer
Hemosiderin

ID what’s at the tip of the pointer
Anthracosis

ID what’s at the tip of the pointer
Anthracosis

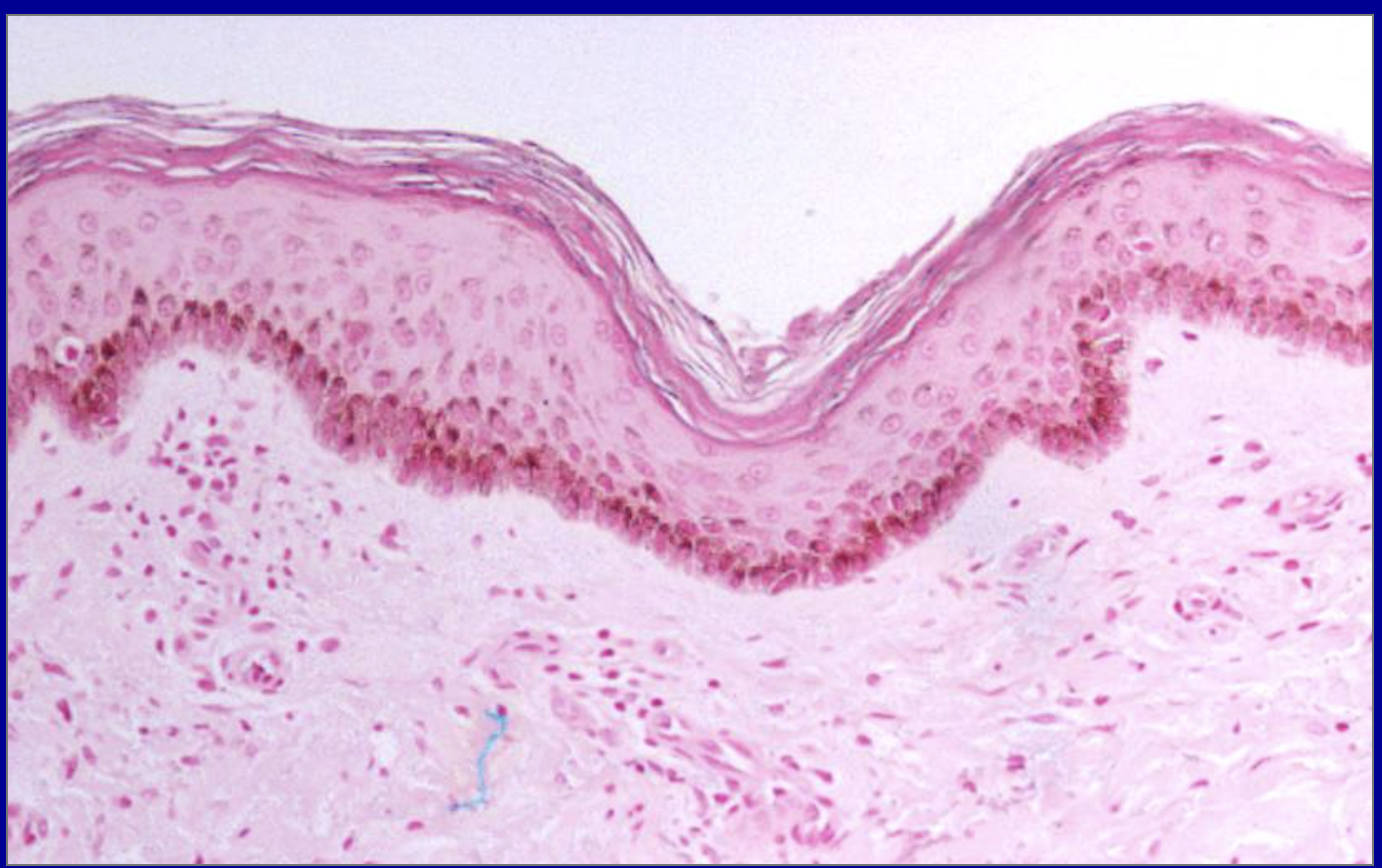
ID the pathology
Metaplasia
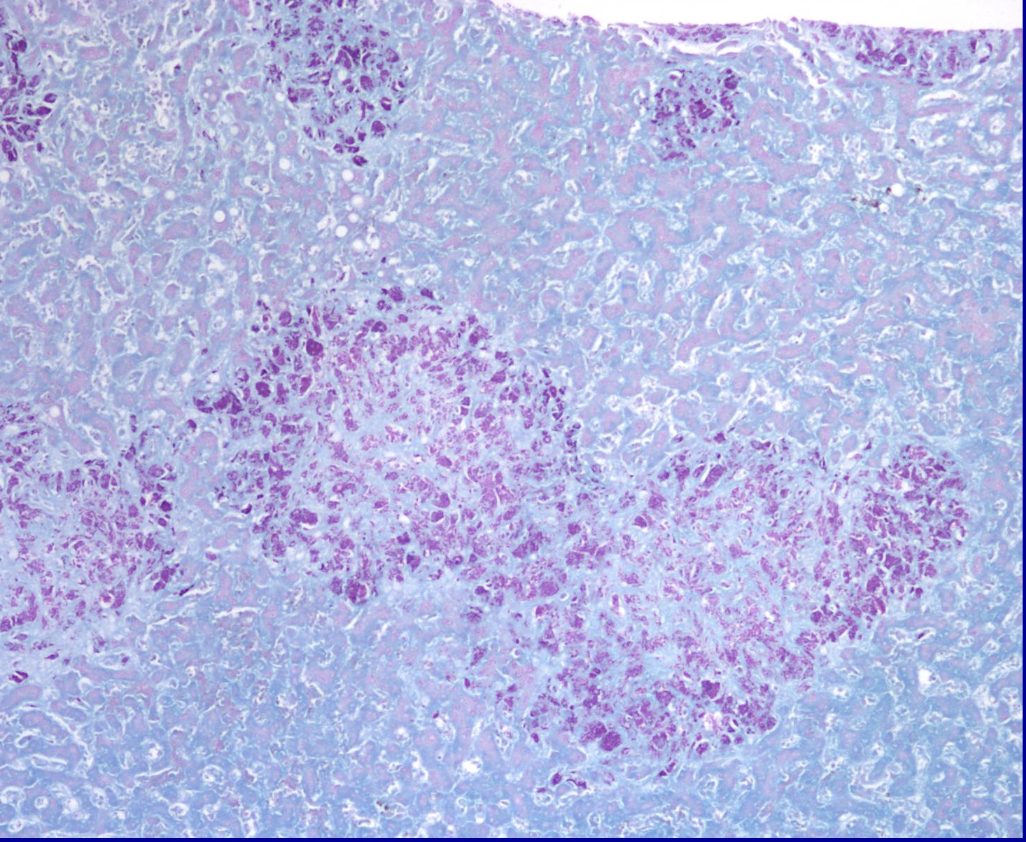
ID the stain
AFB (Acid fast bacillus)
(Zeihl-Neelson)
What is AFB (Acid fast bacillus)
(Zeihl-Neelson) stain used to visualize?
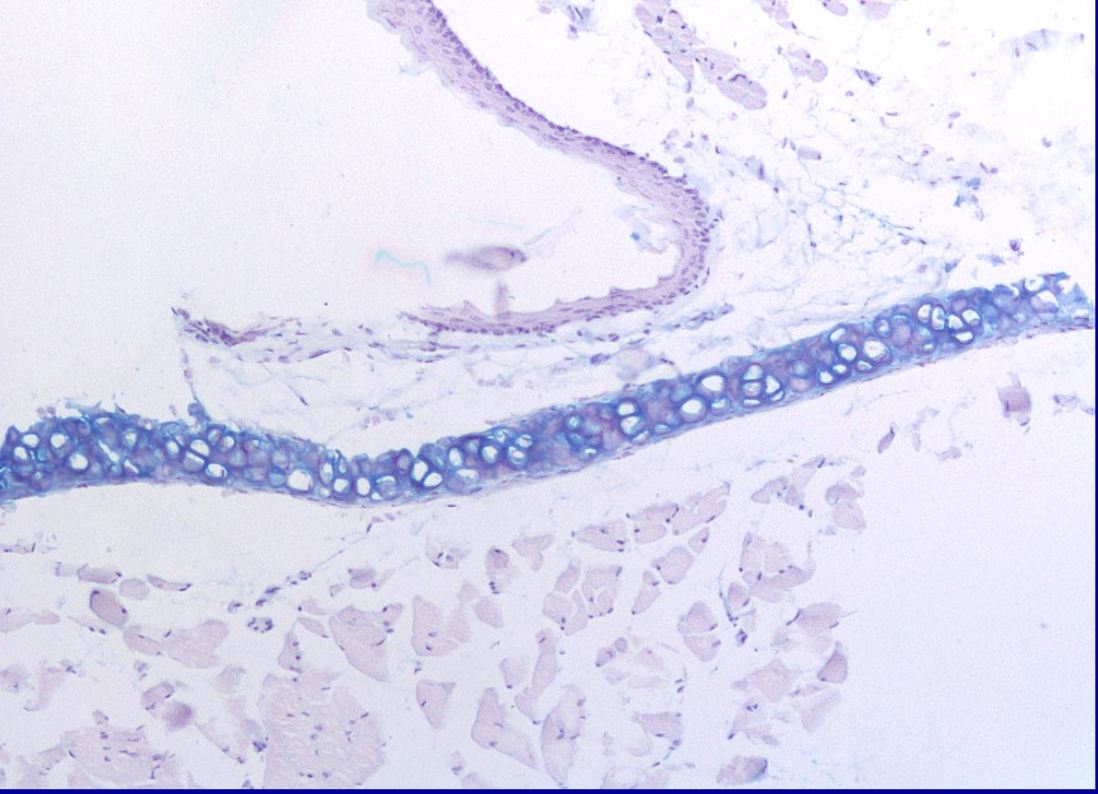
ID the stain type
Alcian blue
What is alcian blue used to stain for?
Specifically detects the acidic polysaccharides, primarily sulfated glycosaminoglycans (GAGs) and proteoglycans (like aggrecan), within the extracellular matrix of cartilage, staining these negatively charged molecules blue to visualize cartilage structure and assess its integrity. |
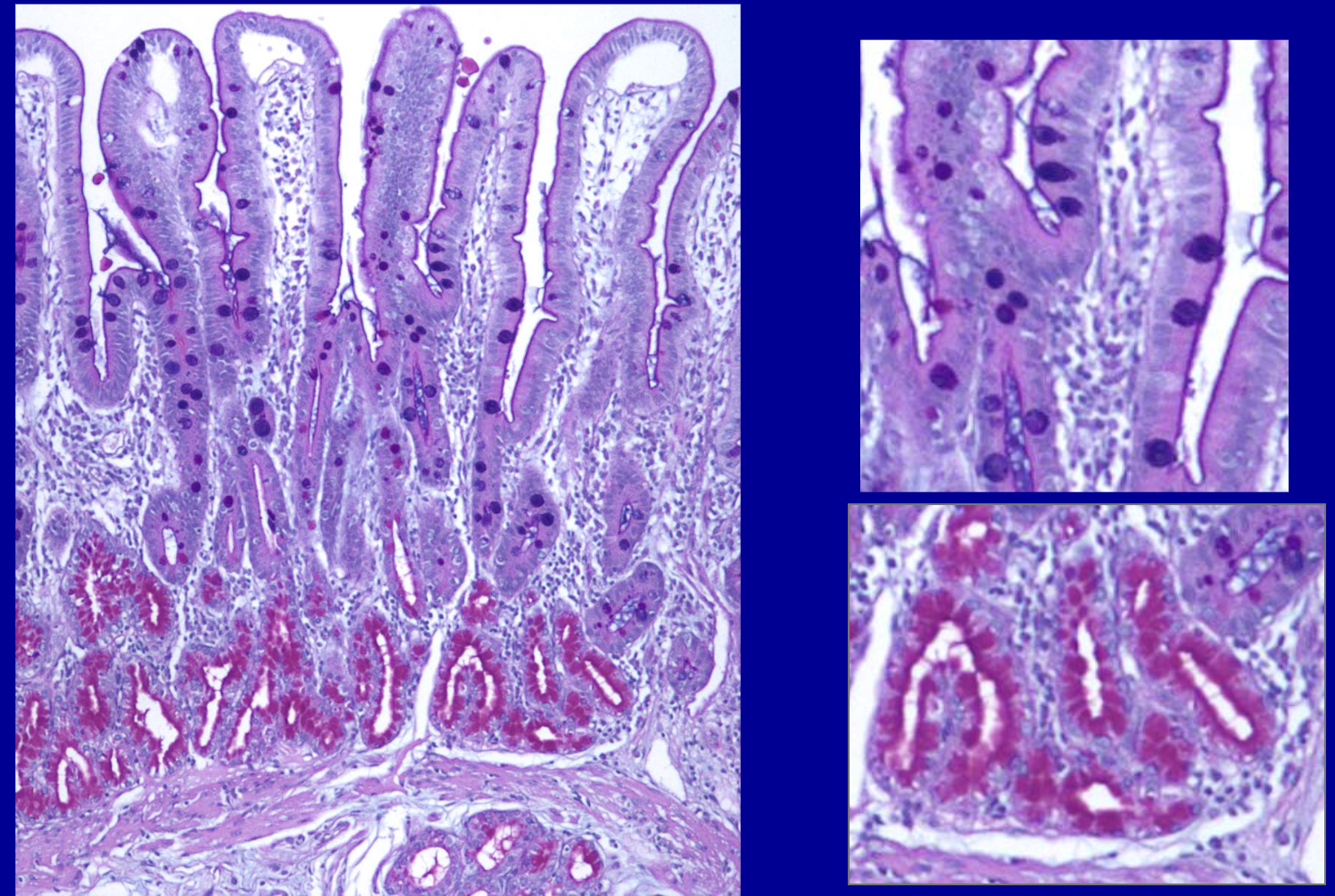
ID the stain
Alcian blue with PAS
What is Alcian blue with PAS used to stain for?
Used to differentiate various carbohydrates from one another.
Ex: Highlights polysaccharides of glycoproteins.
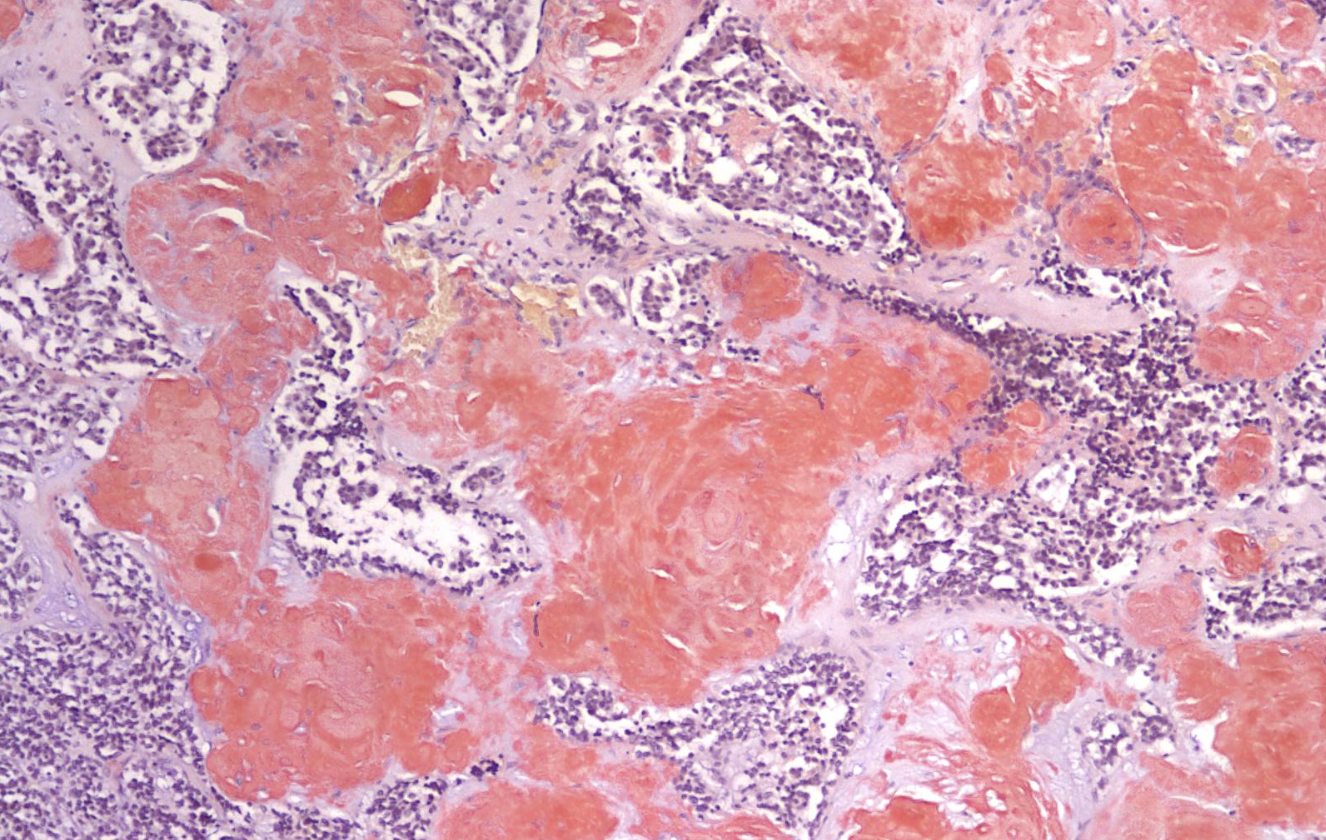
ID the stain type
Amyloid
What is amyloid stain used to detect?
-abnormal protein deposits in tissues -detect the unique β-pleated sheet structure found in amyloid protein fibrils |
What is the GMS
(Gomori Methanamine Silver Stain) used to detect?
Used when fungi are suspected
- Aldehydes on cell wall polysaccharides reduce silver molecules in the stain, which produces a gray/black precipitate
It is used widely as a screen for fungal organisms. It is particularly useful in staining carbohydrates. It can be used to identify the yeast-like fungus Pneumocystis jiroveci, which causes a form of pneumonia called Pneumocystis pneumonia (PCP) or pneumocystosis.
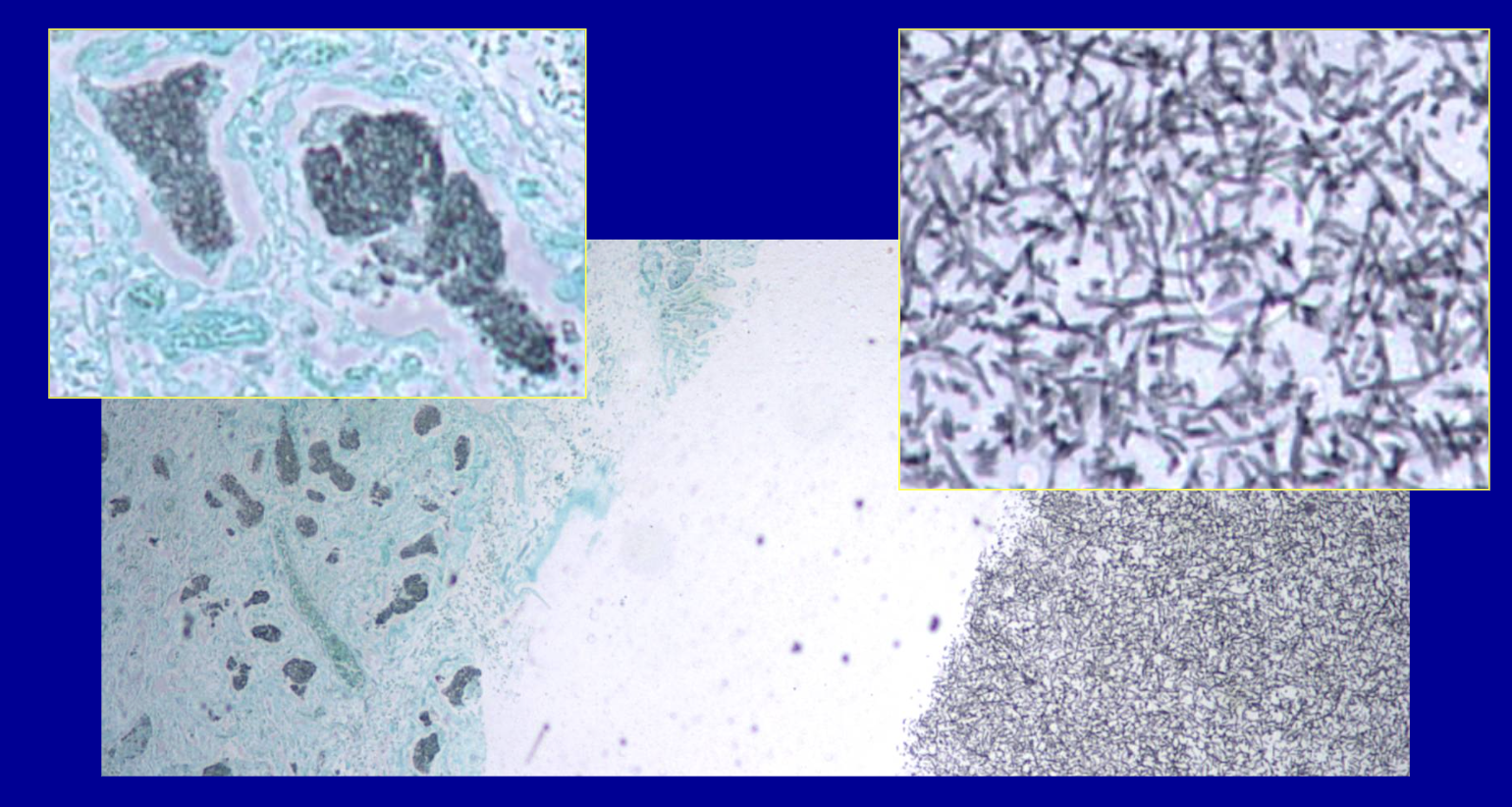
ID the stain type
GMS
(Gomori Methanamine Silver Stain)
pneumonia fungus
ID the stain type
Gram stain
What is the gram stain used to detect?
- this stain detects the thickness and composition of the peptidoglycan layer of bacterial cell walls
- gram-positive bacteria have a thick peptidoglycan layer which stains purple
- gram negative bacteria have a thin peptidoglycan layer which stains pink
It is used to identify the presence and type of bacterial infection in body fluids, tissues, and cultures, allowing for prompt, targeted antibiotic treatment
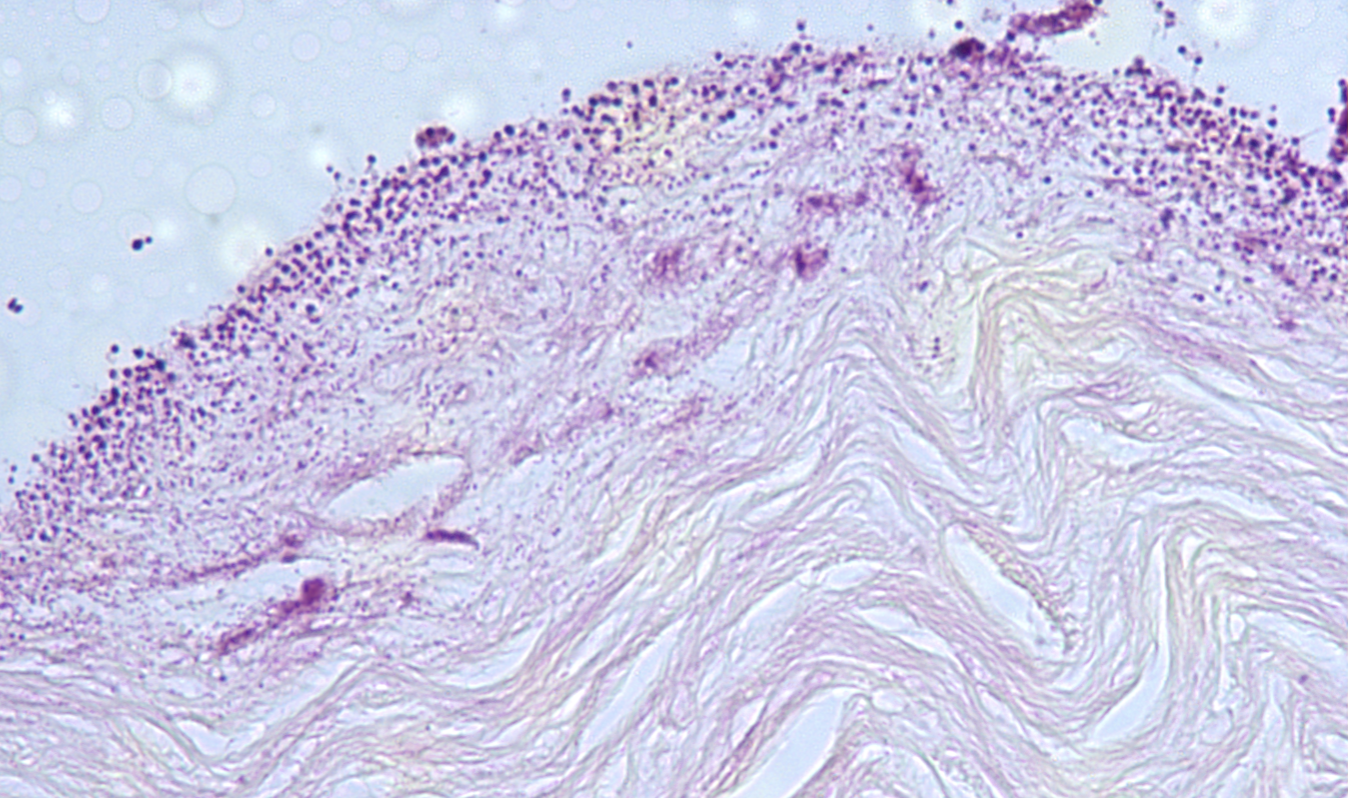
ID the stain type
Jones stain
What is the Jones stain used to detect?
- designed for detection of basement membranes
- uses silver staining to make them black
- can stain reticular fibers but that’s not a main use
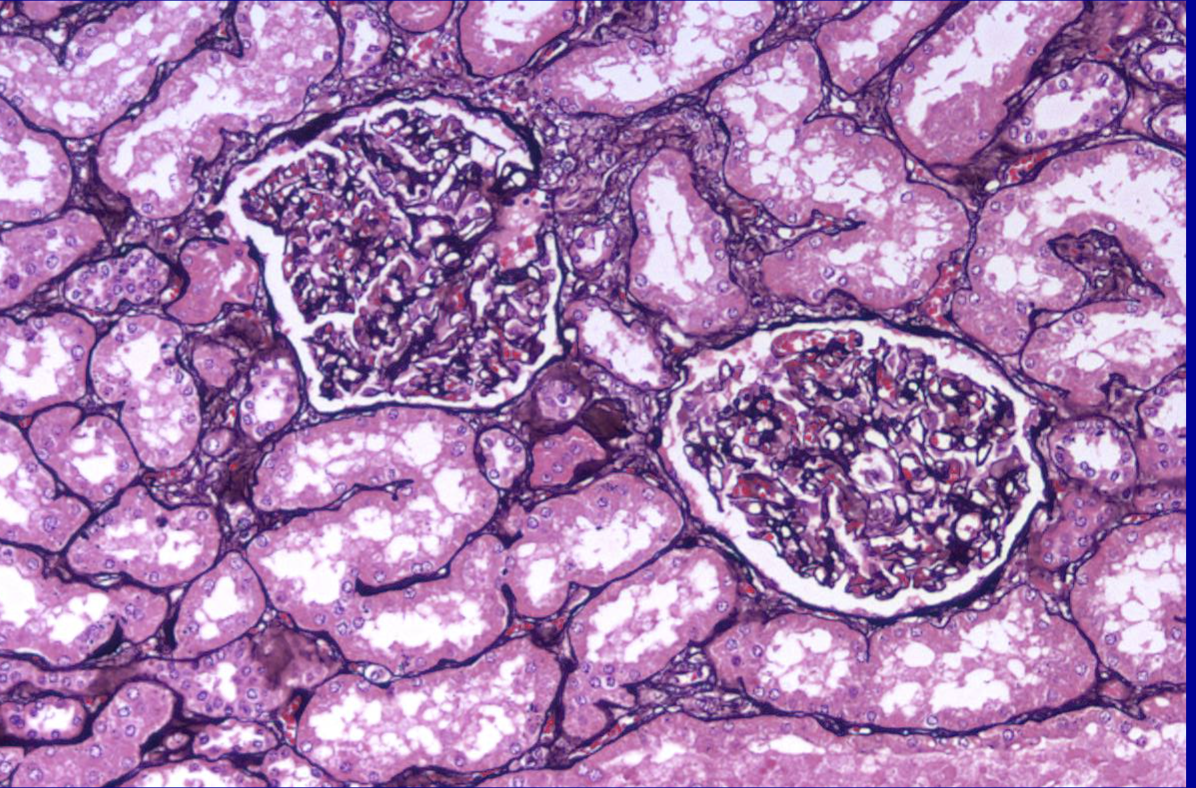
ID the stain type
Melanin stain
What is melanin used to detect?
It targets melanin granules in the cells which have strong reducing properties which reduce silver nitrate and appear dark
primarily used to detect and visualize the pigment melanin in tissue sections, especially when it is present in small amounts or in pigmented, amelanotic tumors.
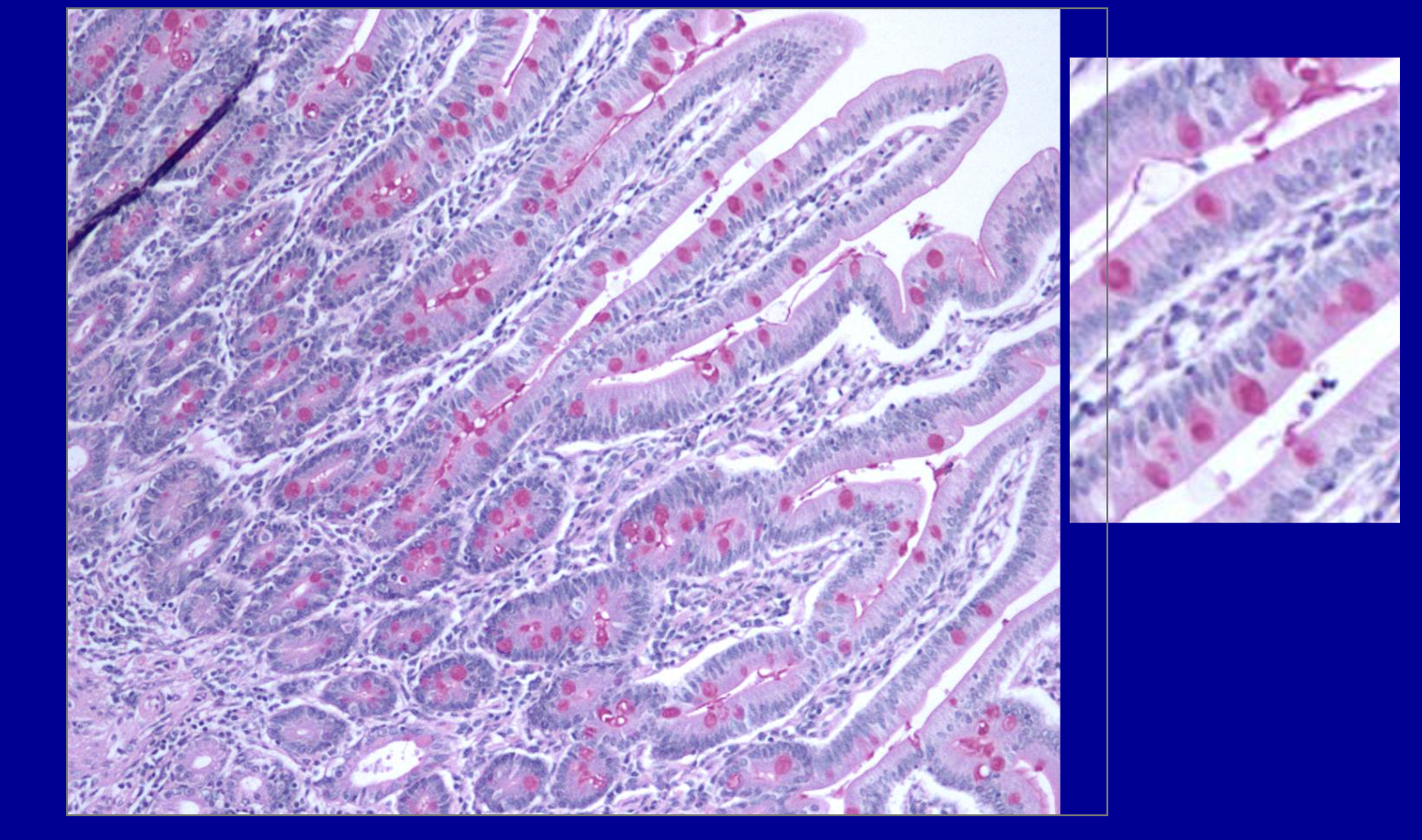
ID the stain type
Muci-carmine stain
What is the Muci-carmine stain used to detect?
Mucicarmine stain is used in pathology to detect epithelial mucins (acid mucopolysaccharides) and the capsule of the fungus Cryptococcus neoformans. It is primarily utilized to distinguish mucin-producing adenocarcinoma from other tumor types (like squamous cell carcinoma) and to highlight signet-ring cell carcinomas.
Highlights mucin by binding to its acidic mucopolysaccharides. Provides better contrast for mucin than other stains (hematoxylin etc)
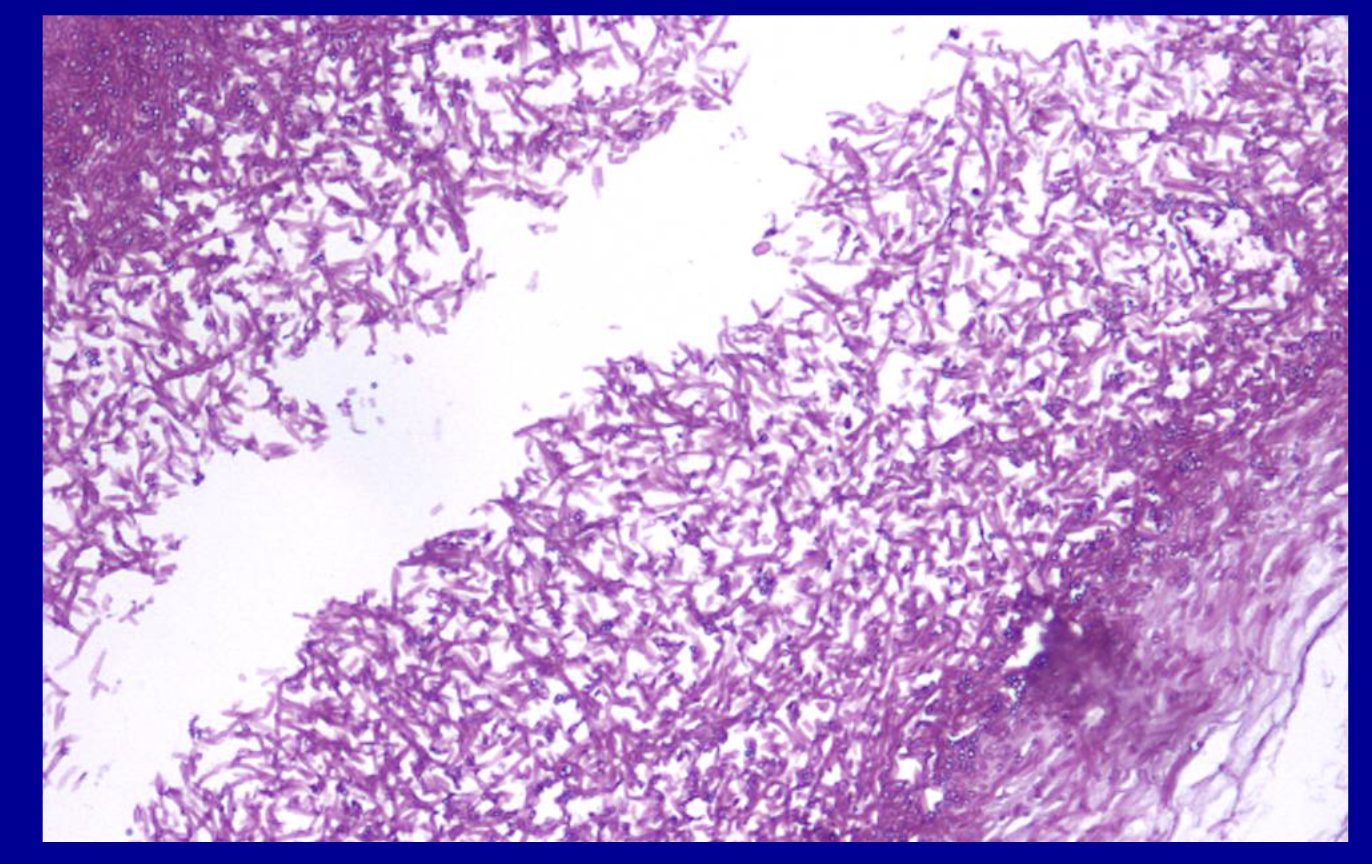
ID the stain type
PAS fungal
What is the PAS fungal stain used to detect?
Highlights polysaccharides such as glycogen and mucous substances like glycoproteins and glycolipids with a bright magenta compound.
The PAS (Periodic Acid-Schiff) fungal stain is primarily used in pathology to detect fungal elements—specifically hyphae, yeasts, and spores—in tissue samples, skin scrapings, and nail clippings by staining their carbohydrate-rich cell walls a vibrant magenta. It is crucial for diagnosing superficial and systemic mycoses (e.g., Candida, Aspergillus).
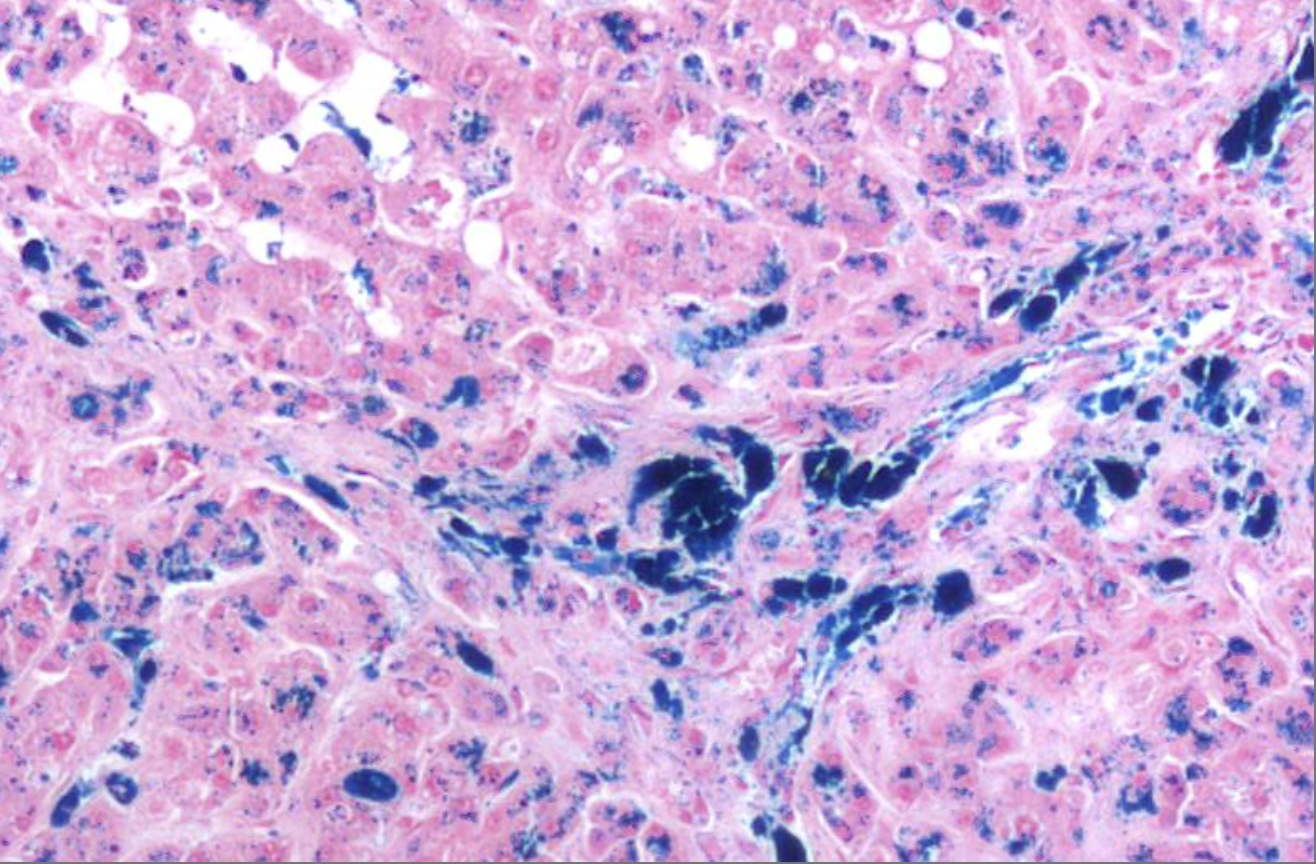
ID the stain type
Prussian Blue - Iron stain
What is the iron stain - prussian blue - used to detect?
Used to detect ferric iron in tissue sections for diagnosing iron overload disorders like hemochromatosis. Usually it marks hemosiderin, a form of stored iron, especially in macrophages and hepatocytes to indicate normal iron metabolism or iron overload.
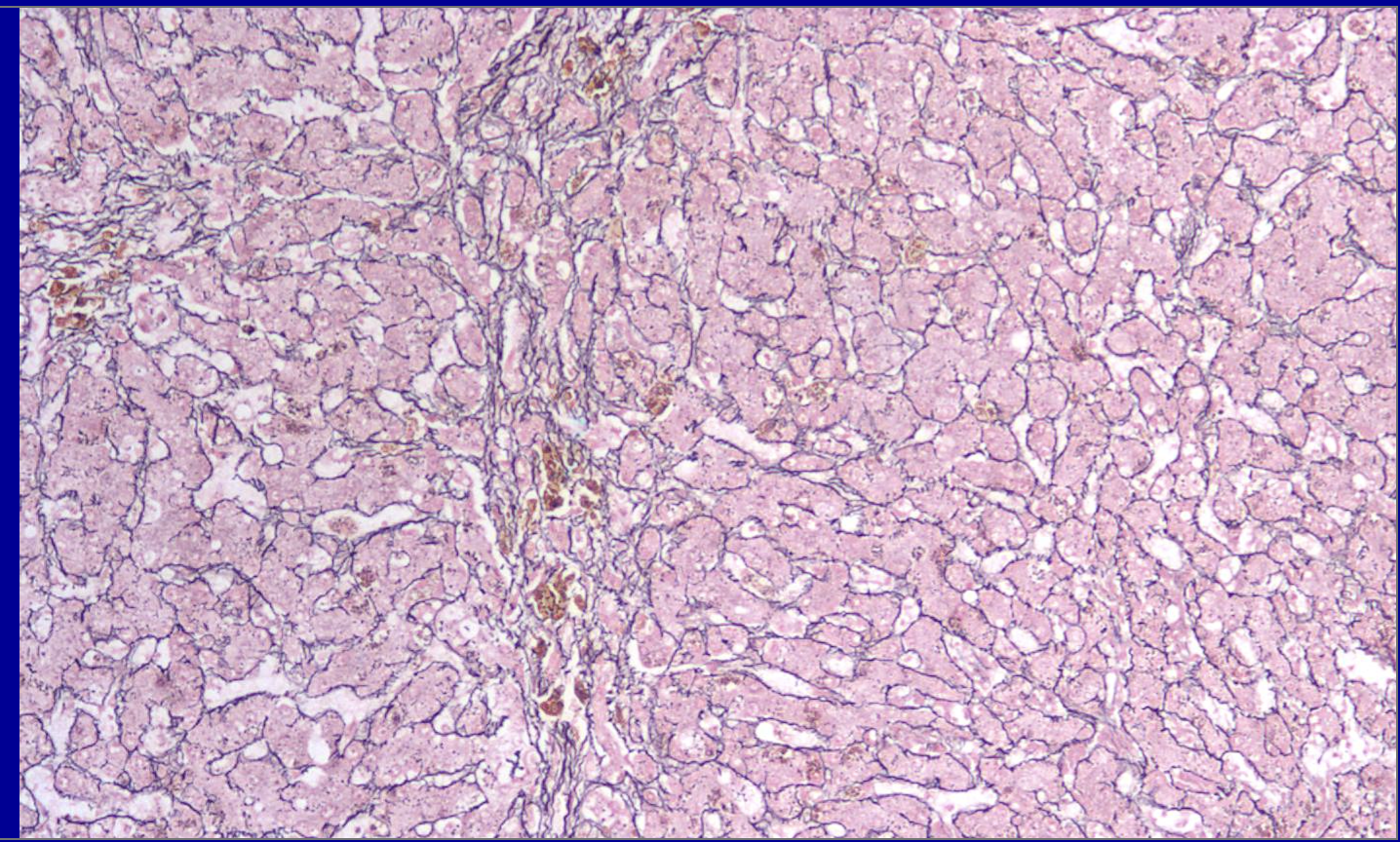
ID the stain type
Reticulum (reticulin) stain
What is Reticulum (reticulin) stain used to detect?
Reticulum (reticulin) stains are specialized histological techniques, typically using silver impregnation (e.g., Gordon and Sweet’s method), designed to detect and visualize reticular fibers (Type III collagen) in tissue samples. These fibers form a fine, delicate meshwork that provides structural support to soft tissues.
In pathology, these stains are primarily used to evaluate tissue architecture, fibrosis, and stromal changes.
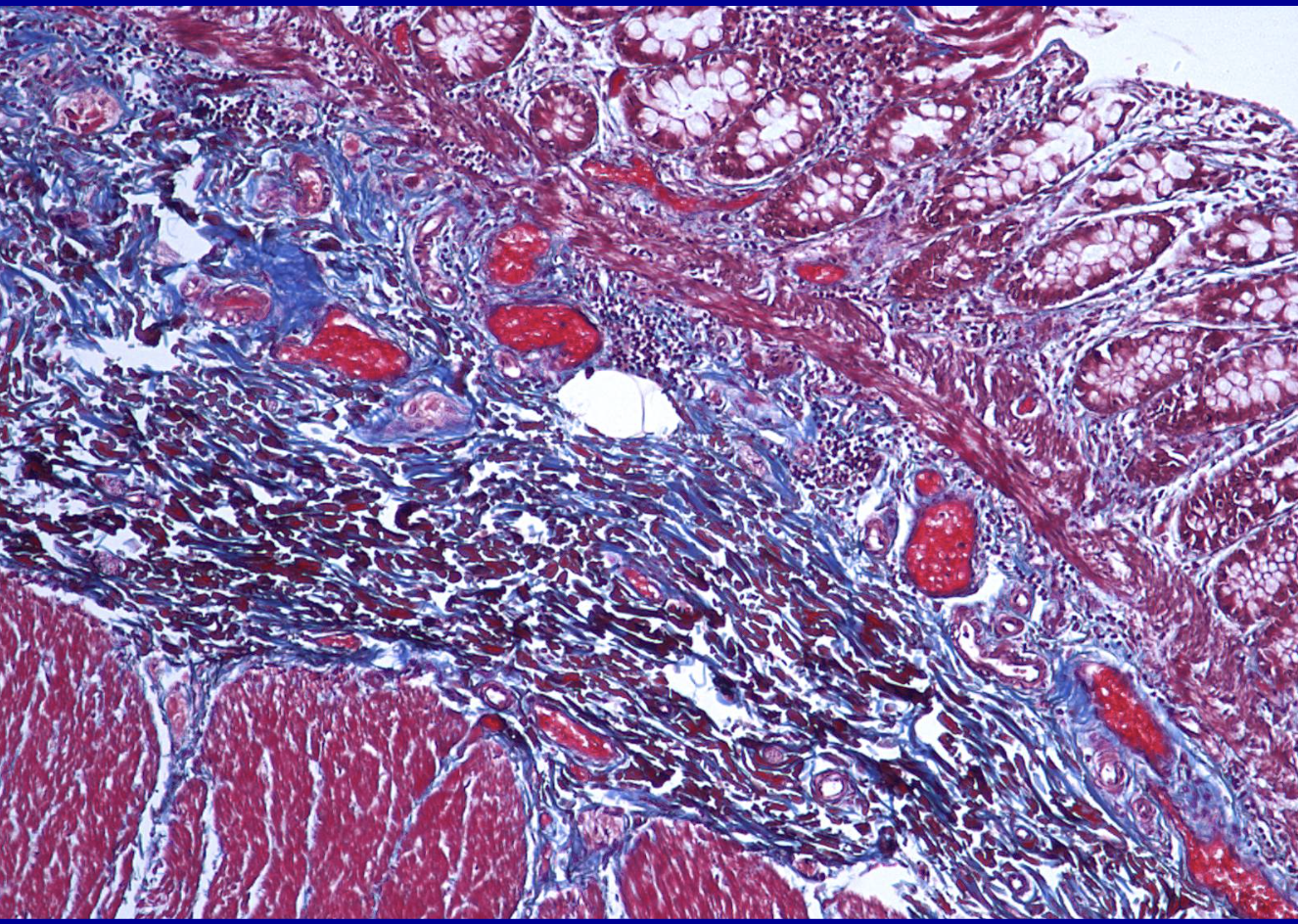
ID the stain type
Trichrome
What is the trichrome stain used to detect?
trichrome stain is primarily used in pathology to detect and visualize fibrosis (excessive collagen deposition) and differentiate between collagen fibers (blue/green), muscle fibers/cytoplasm (red), and nuclei (black). It is essential for assessing tissue remodeling, chronic liver disease (cirrhosis), kidney glomerular fibrosis, and cardiac dama
Uses 3 dyes to show structure (collagen stains blue, cytoplasm/muscle → red, nuclei → dark blue/black)
Trichrome stains are designed to distinguish connective tissue, primarily since collagen stains blue in contrast to red muscle and cytoplasm
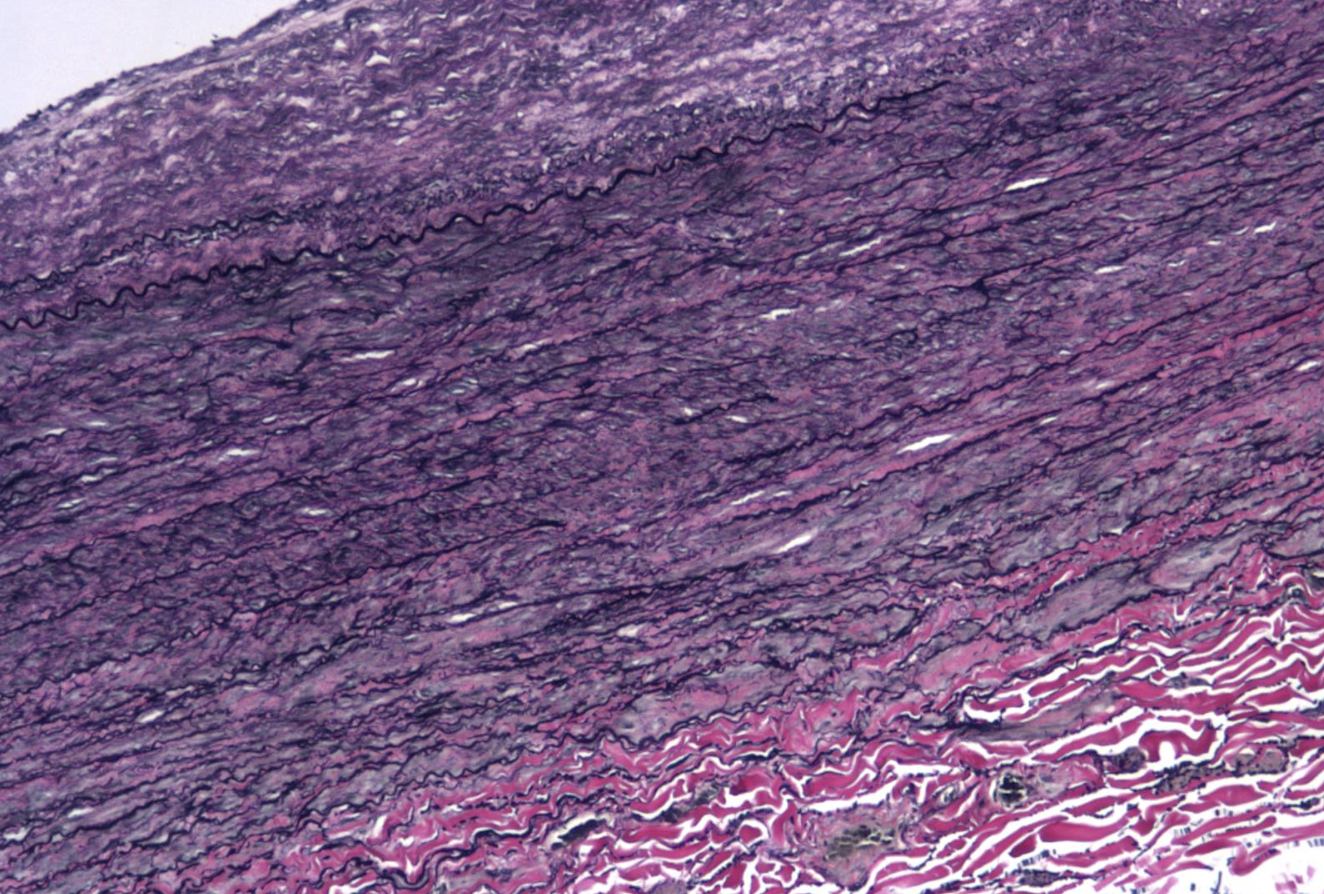
ID the stain type
VVG (Verhoeff- Van Gieson) (elastin stain)
What is the VVG (Verhoeff- Van Gieson) (elastin stain) used to detect?
The Verhoeff-Van Gieson (VVG) stain is a histological technique used to visualize and detect elastic fibers (stained blue-black) and differentiate them from collagen (red) and other tissues (yellow). It is primarily used to identify vascular diseases (e.g., arteriosclerosis), lung pathologies (e.g., emphysema), and to assess tumor invasion or connective tissue integrity.
What is the name of 3 different “free radicals” (ROS)?
Hydroxyl radical, hydrogen perioxide, and superoxide anion
Is oxygen required for glycosis?
No
Is oxygen required for aerobic respiration?
Yes
What is the starting substrate of glycosis?
Glucose
What is the starting substrate of aerobic respiration?
Pyruvate
What is the end product of glycolsis?
Pyruvate
What is the end product of aerobic respiration?
Carbon dioxide (CO2) + H2O + ATP
Where are the enzymes of glycolysis located?
Cytosol
Where are the enzymes of aerobic respiration located?
Mitochondria
What is dystrophic pathological calcifcation characteriszed by?
Damanged tissue, normal calcium amounts
What is metastatic pathological calcifcation characterized by?
Normal tissue, high calcium amounts
What are four mechanisms cells used for inactivating free radicals (ROS)?
Superoxide dismutase, catalase, glutathione peroxidase, antioxidants
What are the four major causes of inflammation?
Infections, tissue necrosis, foreign bodies, persistent immune reactions
What are the four cardinal signs of inflammation?
Redness (rubor) caused by vasodilation, heat (calor) caused by increased blood flow, swelling (tumor) caused by increased vascular permeability, pain (dolor) caused by chemical mediators
What are the two main vascular changes in acute inflammation?
Vasodilation and increased vascular permeability
What is the defintion of exudate?
An extracellular fluid that has a high protein content and contains cellular debris
What is the defintion of transudate?
A fluid with low protein content and cellular fluid
What is necrosis?
A type of cell death in which the cell membrane breaks down, causing enzymes to leak out and digest the cell. This process triggers inflammation and usually occurs when cell injury is severe and cannot be reversed.
What is apoptosis?
A controlled form of cell death in whic a cell breaks down its own DNA and proteins and seperates into small fragments. The cell membrane stays intact, allows teh fragments to be safely removed without causing inflammation.
What is hypoxia?
A condition in which tissues do not recieve enough oxygen to meet their metabolic needs. it can result form poor oxygenation of the blood, reduced oxygen-carrying capacity, or carbon monoxide poisioning.
What is ischemia?
A reduction in blood flow/supply to tissues. Leads to decreased oxygen delivery and essential nutrients and a build up of toxic metabolic waste.
What are ROS/free radical species?
What is hypertrophy?
A increase in the size of existing cells, which causes an organ to become larger without an increase in cell number.
What is hyperplasia?
An increase in the number of cells in a tissue or organ due to increased cell division, leading to organ enlargement.
What is atrophy?
A decrease in the size of cells due to loss of cell substance, which can cause a tissue or organ to shrink.
What is metaplasia?
A reversible change in one mature cell type is replaced by another mature cell type that is better able to withstand stress. This occurs through reprogramming of stem cells rather than direct transformation of existing cells.
What is pyknosis?
Early nuclear change in cell death characterized by nuclear shrinkage and condensation of DNA into a dark, dense mass.
What is karolysis?
The final stage in cell death in which the nucleus fades and disappears due to enzymatic digestion of DNA (ghost cell, looks like vaculated empty space).
What is karorrhexis?
Middle stage in cell death that occurs when the pyknotic nucleus fragments into multiple pieces as the DNA breaks apart (nuclear dust).
What is the P-450 mixed oxygenase system (smooth ER)?
This system is a drug-metabolising enzyme system in the smooth ER mainly in the liver that oxidizes drugs, toxins, and endogenous compounds by making substances more polar to be easily removed
What are examples of vasoactive amines?
Histamine, serotonin,
What are some effects of vasoactive amines?
Vascular changes
Where are vasoactive amines stored/produced? What are they produced in response to? Are they systemic or local?
Stored in cells, local, particuarly mast cells, basophils, and platelets. In response to physical injury, mast cell Ig, complement. Local
What are examples of arachidonic acid metabolite chemical mediators?
Prostaglandins, leukotrienes
Are arachidonic acid metabolites systemic or local? What are they produced by?
Local/systemtic, produced by macrophages, white blood cells, endothelial cells
What are some effects of arachidonic acid metabolites?
Vascular and cellular changes, pain, fever.
What are some examples of cytokine/chemokine chemical mediators?
IL, TNF, Interleukins, chemokines
Are cytokines/chemokines local or systemic? What are they produced by?
Local/systemic, produced by macrophage, white blood cells, endothelial cells
What are some effects of cytokines/chemokines?
Local endothelial activation, systemic acute phase response (fever)
What are examples of complement chemical mediators?
Complement cascade
Is completement local or systemic? What is is produced by?
Systemtic, produced by liver, activated by protease
What are some effects of complement?
Opsonization, white blood cell recruitment, MAC (membrane attack system complex)(lysis), inflammation (stimulate release of histamine from mast cells)
What does complement bind to?
C1 binds to Ag-Ab, C1 binds to microbe surface molecule
Is platelet activating factor local or systemic? What is it produced by?
Local, produced by leukocytes and endothelial cells
What are some effects of platelet activating factor?
All aspects of inflammation
What is an example of a product of coagulation chemical mediator?
Thrombin
Is thrombin/products of coagulation local or systemic? What is it produced by?
Systemic, produced by liver
What are some effects of thrombin?
Blood clotting
What is an example of a kinin chemical mediator?
Bradykinin
Is kinin/bradykinin local or systemic? What is it produced by?
Systemic, produced by liver
What are some effects of bradykinin?
Vasoactive, pain
What are some examples of neuropeptide chemical mediators?
Substance P
Are neuropeptides/substance P local or systemic? What is it produced by?
Local, produced by nerve fibers
What are some effects neuropeptides/substance P?
Vascular changes, pain