All Equations
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
59 Terms
what is the mass number?
the total number of protons and neutrons in a nucleus
how do you work out the number of neutrons?
mass number - atomic number
how do you work out the moles from a mass and Mr?
mass (g) / Mr (gmol-1)
how do you work out the moles from a volume and concentration?
volume x concentration
how do you work out the moles from a gas volume?
volume (dm3) / 24
volume (cm3) / 24000
what is the ideal gas equation?
pV = nRT
p = pressure (Pa)
V = volume (m3)
n = mol
R = gas constant (8.314 Jmol-1)
K = temperature (K)
acid + carbonate
salt + water + carbon dioxide
acid + metal oxide
salt + water
acid + alkali
salt + water
acid + metal
salt + hydrogen
write an equation for the first ionisation energy of magnesium
Mg (g) -> Mg+ (g) + e-
how do you calculate enthalpy change of reaction using average bond enthalpies?
ΔH = (sum of bond enthalpies of reaction) - (sum of bond enthalpies of products)
what is the equation used to calculate rate?
rate = change in conc/time
for the reaction below, deduce an expression for Kc:
2[A] + 3[B] + [C] ⇌ [D] + 4[E]
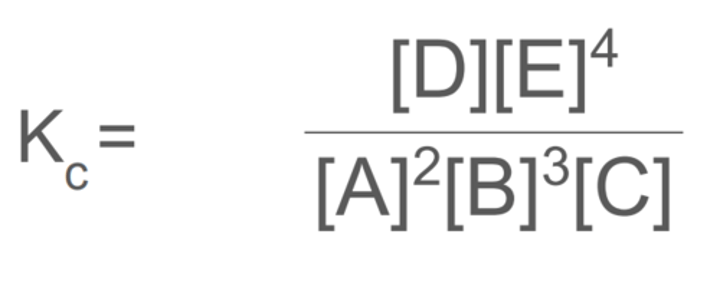
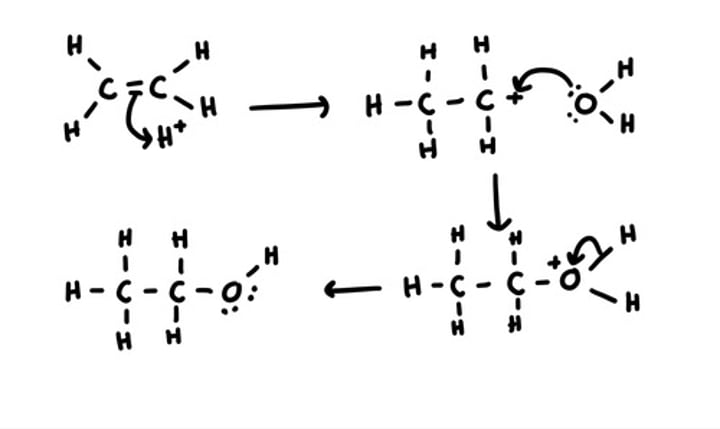
draw a mechanism for the addition of water to ethene
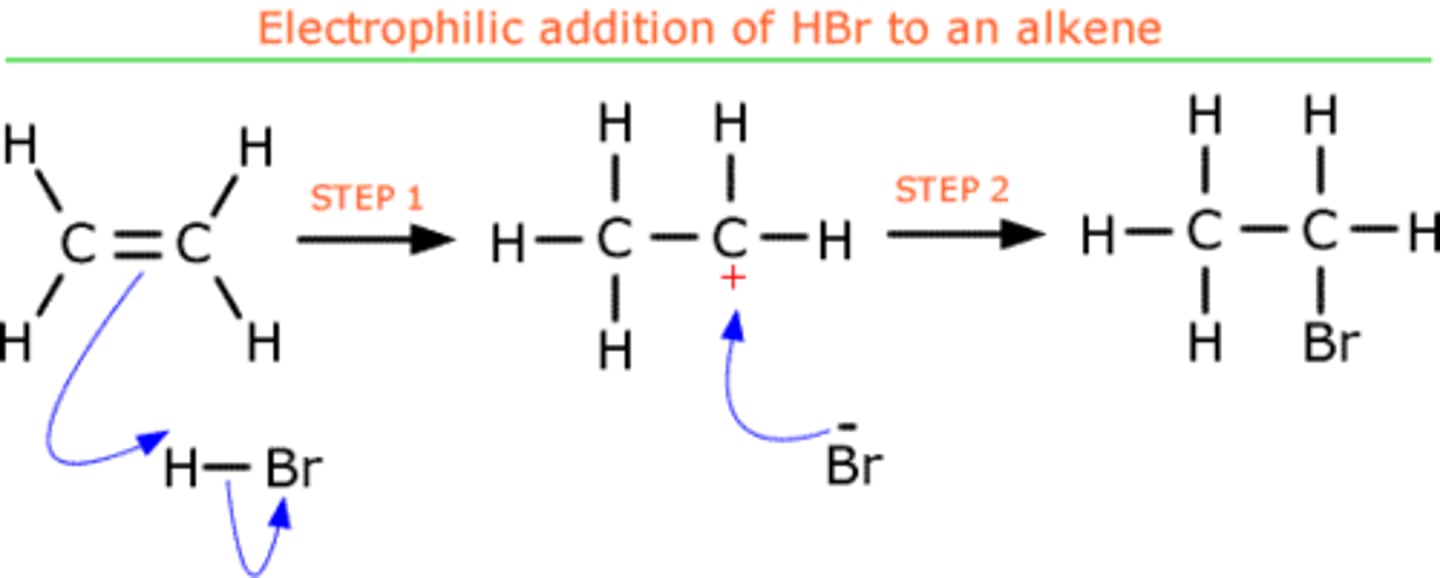
draw a mechanism for the reaction of HBr and ethene
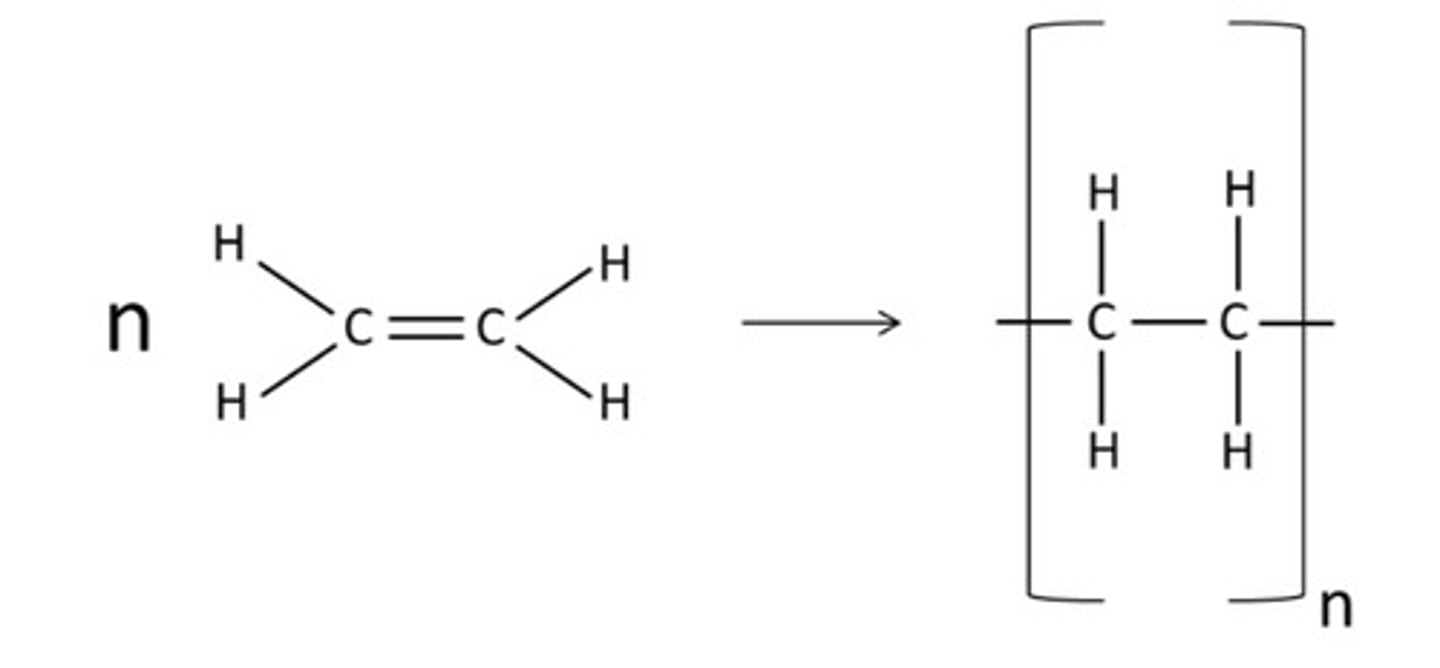
draw how you would represent the polymerisation of ethene
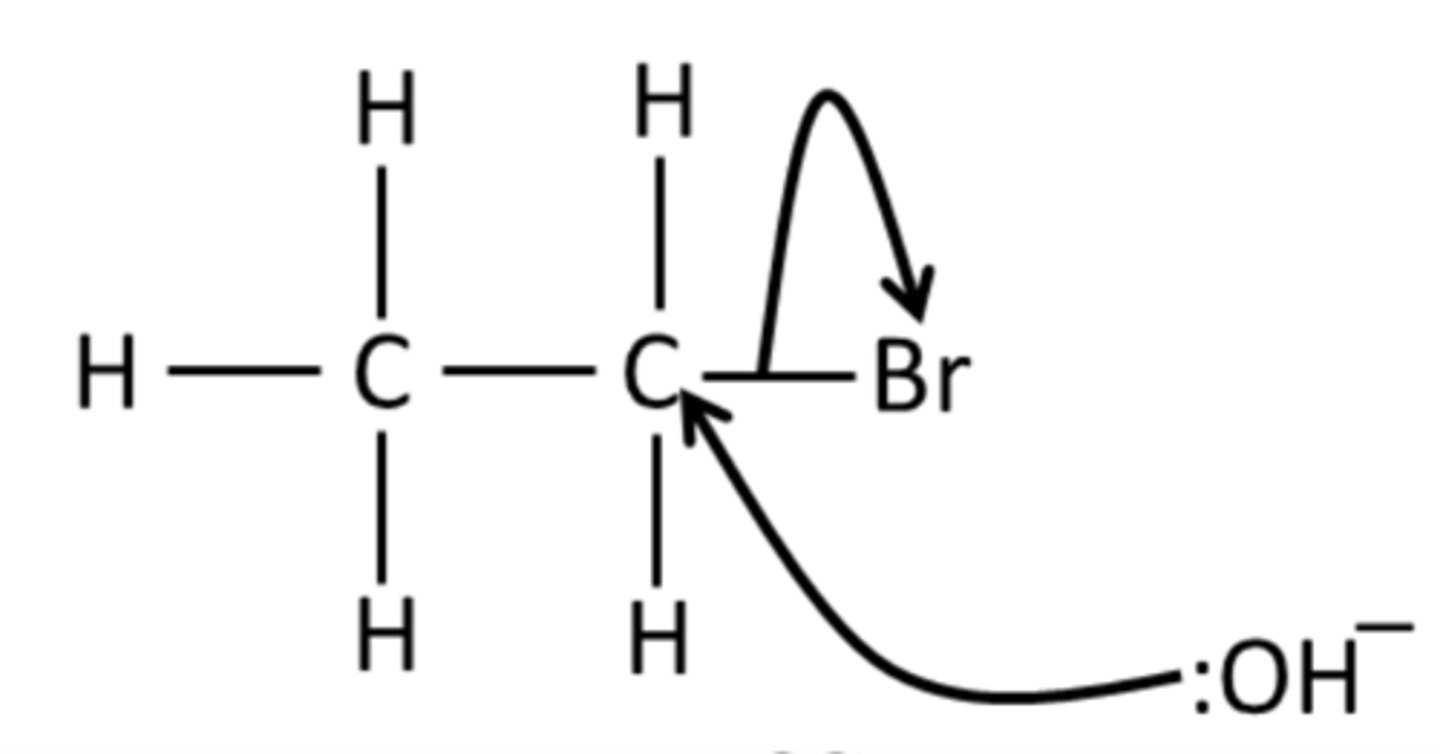
draw the mechanism for the reaction of bromoethane with NaOH (aq)
write an equation for the decomposition of ozone into oxygen
(uv light) Cl2 -> 2Cl
O3 + Cl -> ClO + O2
ClO + O3 -> Cl + 2O2
2O3 -> 3O2
write an equation to show how nitrogen monoxide can decompose ozone
O3 + NO -> NO2 + O2
NO2 + O3 -> NO + 2O2
O3 + O -> 2O2
what is the rate equation?
rate = k[X]^x[Y]^y
k = rate constant
[X] and [Y] are concentrations of species X and Y
x and y are the orders of reaction with respect to X and Y
what is the equation used to determine rate using half life in a first order reaction?
k = ln2/t 1/2
what is the relationship between rate and time?
rate ∝ 1/t
how do you determine the rate constant from a rate concentration graph of first order?
k = rate / concentration
what is the Arrhenius equation? what does each term mean?

how can you convert the Arrhenius equation into a useful term for plotting a graph?
lnk = -Ea/RT + lnA
graph of lnk against 1/T is a straight line
gradient = -Ea/R
y intercept = lnA
mole fraction equation
number of moles of substance A / total number of moles of all substances
partial pressure equation
mole fraction x total pressure
what is the relationship between concentration of a substance and its partial pressure?
concentration of a substance ∝ its partial pressure
write the acid dissociation constant expression
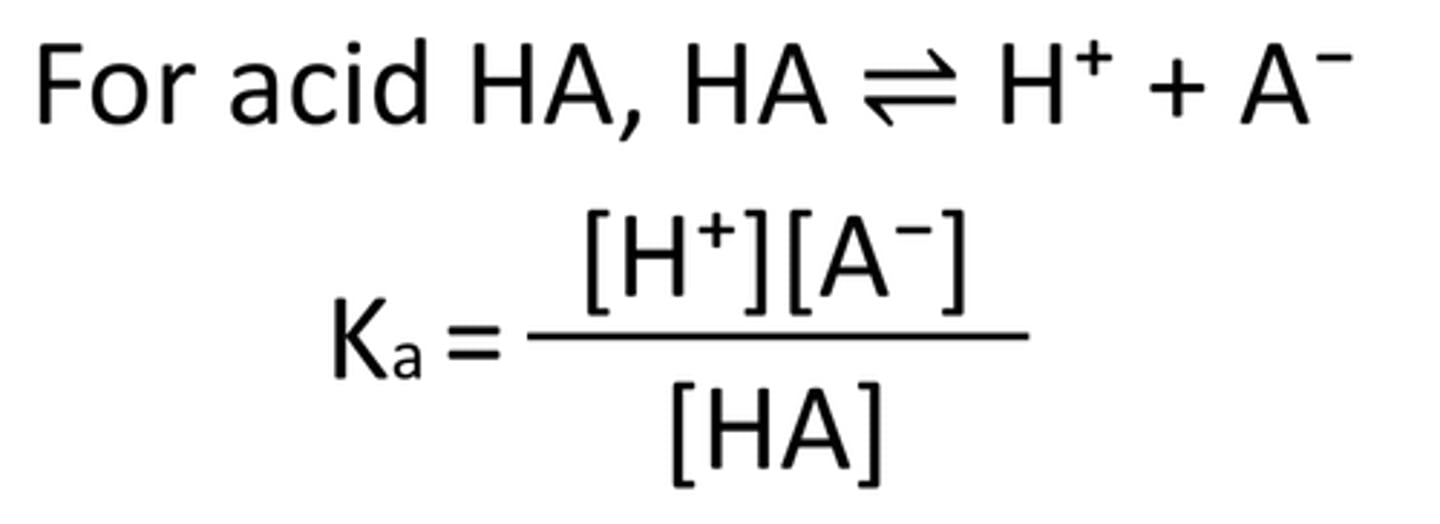
write the equation used to convert Ka into pKa
pKa = -log10Ka
write the equation used to convert pKa into Ka
Ka = 10^-pKa
write the equation used to convert concentration of H+ into pH
pH = -log[H+]
write the equation used to convert pH into concentration of H+
[H+] = 10^-pH
[H+] of a strong acid is equal to what?
[H+] = [HA]
write the equation used to calculate [H+] of weak acids
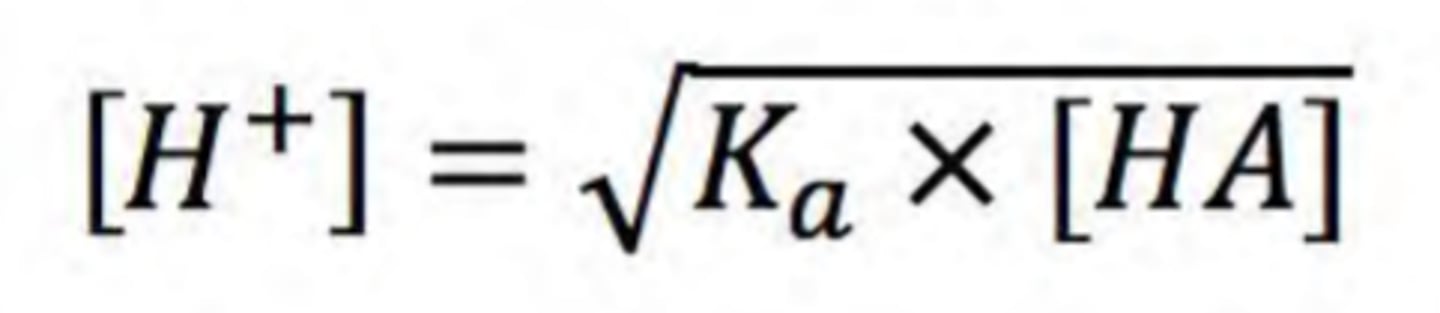
write the expression for the ionic product of water, Kw
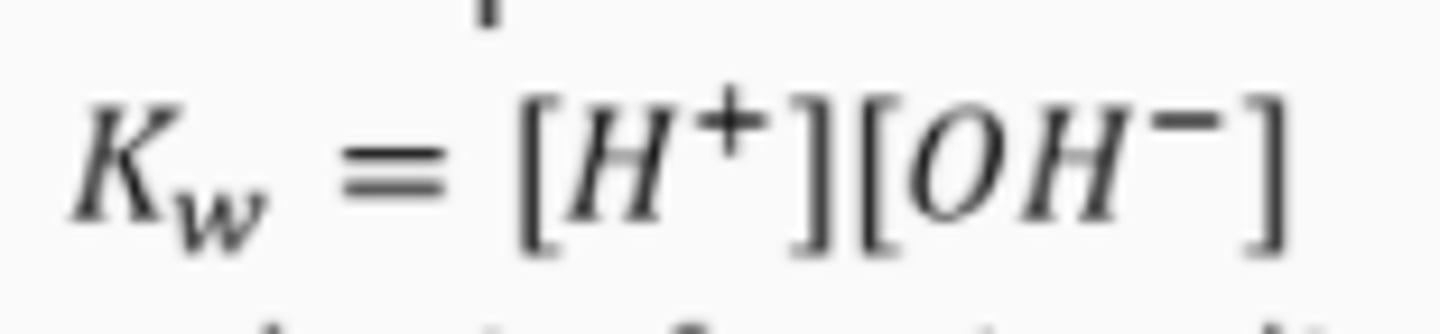
what is the value of Kw at 298K?
1.0 x 10^-14
indices of of [H+] and [OH-] always adds up to what value?
-14
write the equation used to calculate [H+] of strong bases
[H+] = Kw / [OH-]
write the equation used to calculate [H+] of a buffer solution
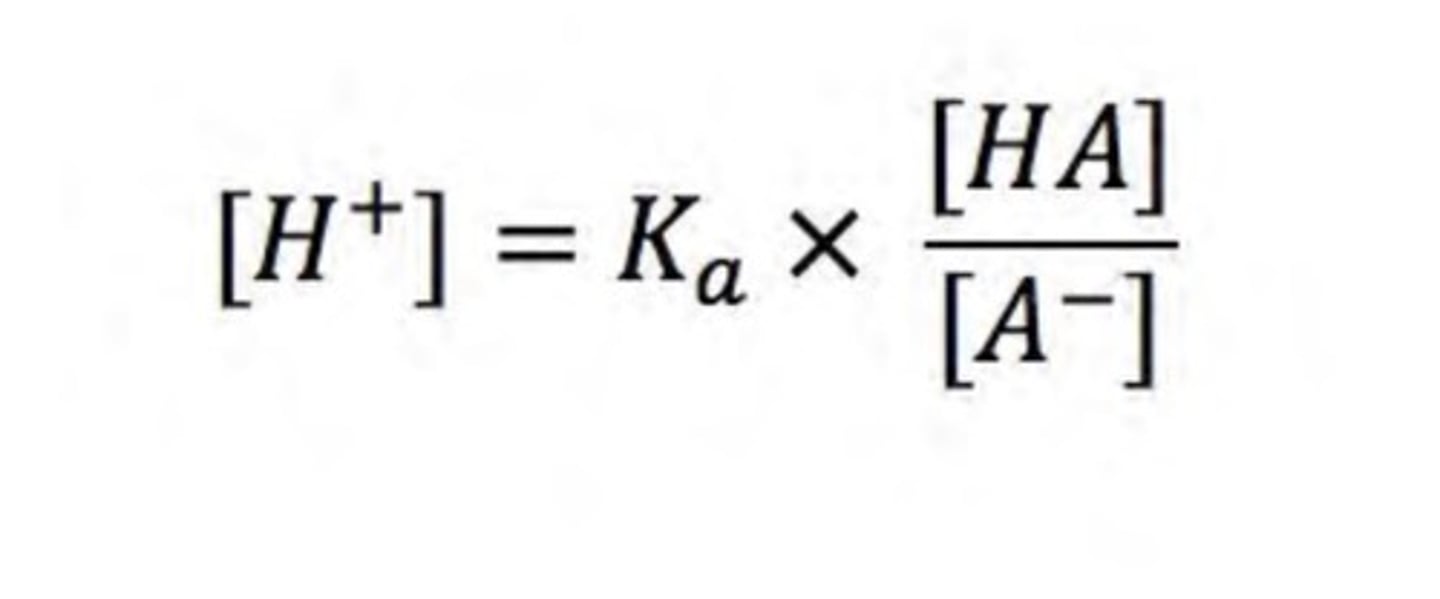
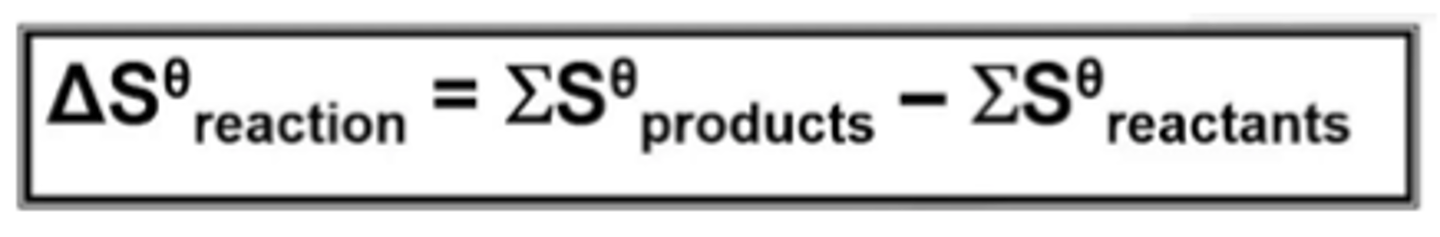
write the equation used to calculate entropy change
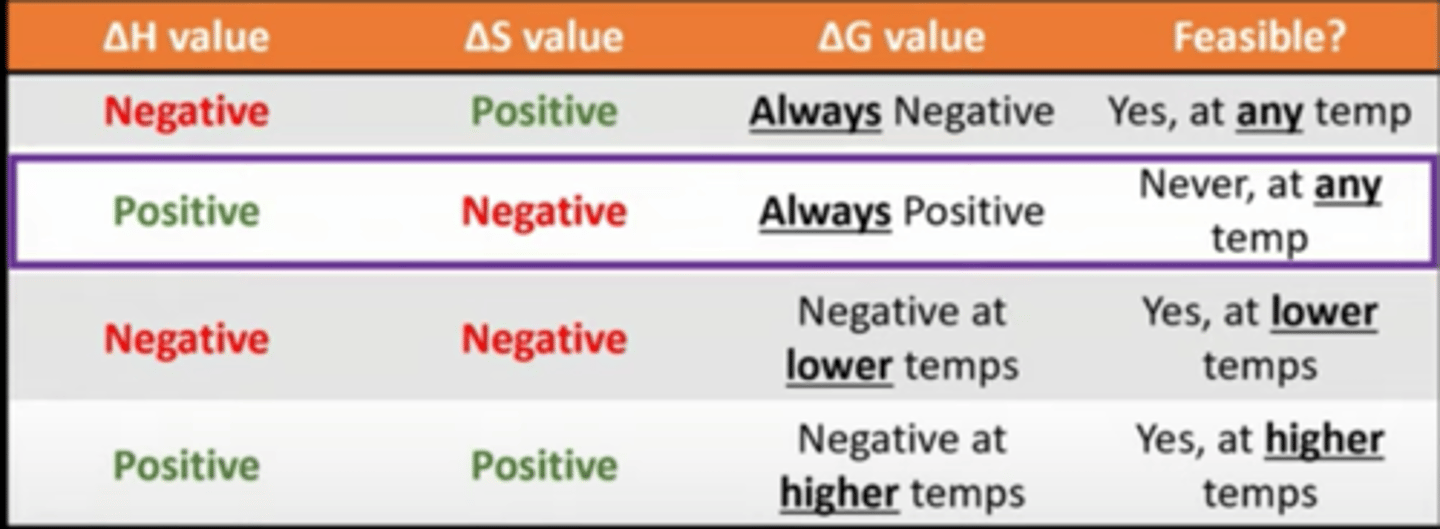
write the Gibbs' free energy equation and state what each symbol means
ΔG = ΔH - TΔS

feasibility of reactions based on the values of each part of the equation
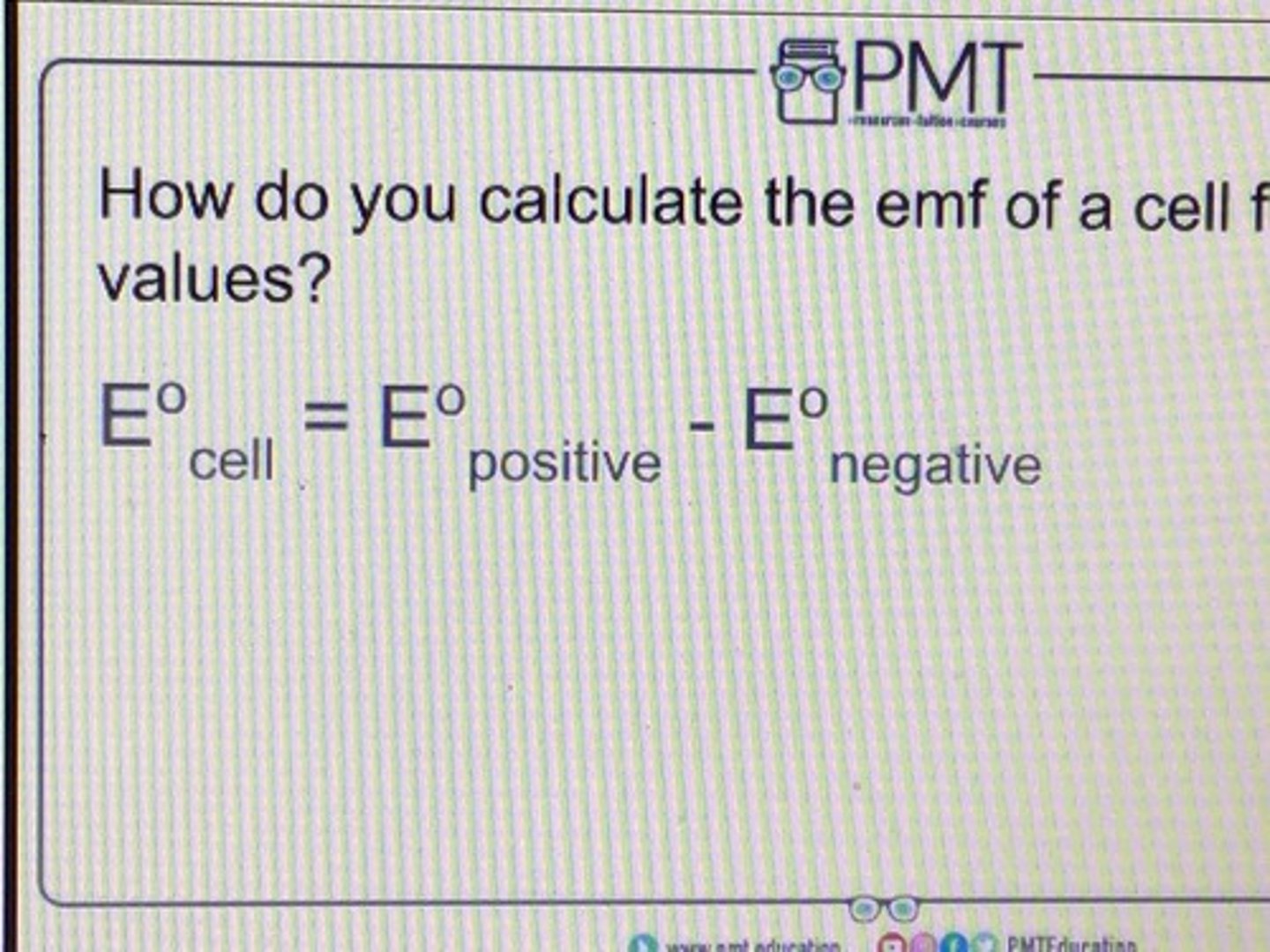
how do you calculate the emf of a cell from E values?
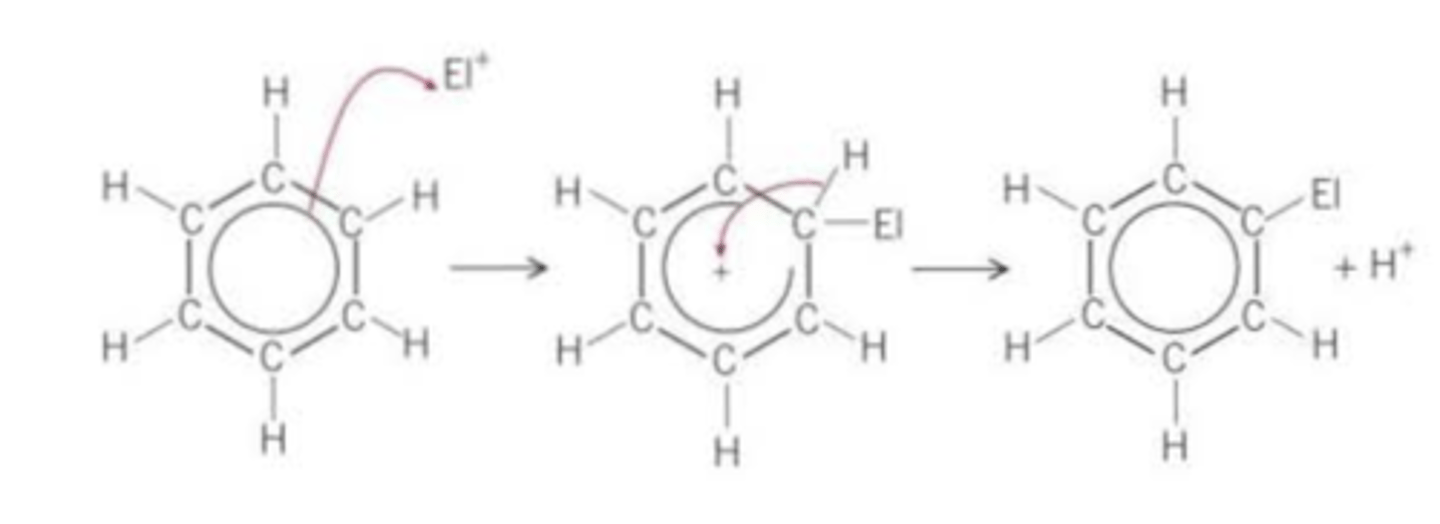
draw a general electrophilic substitution mechanism of benzene
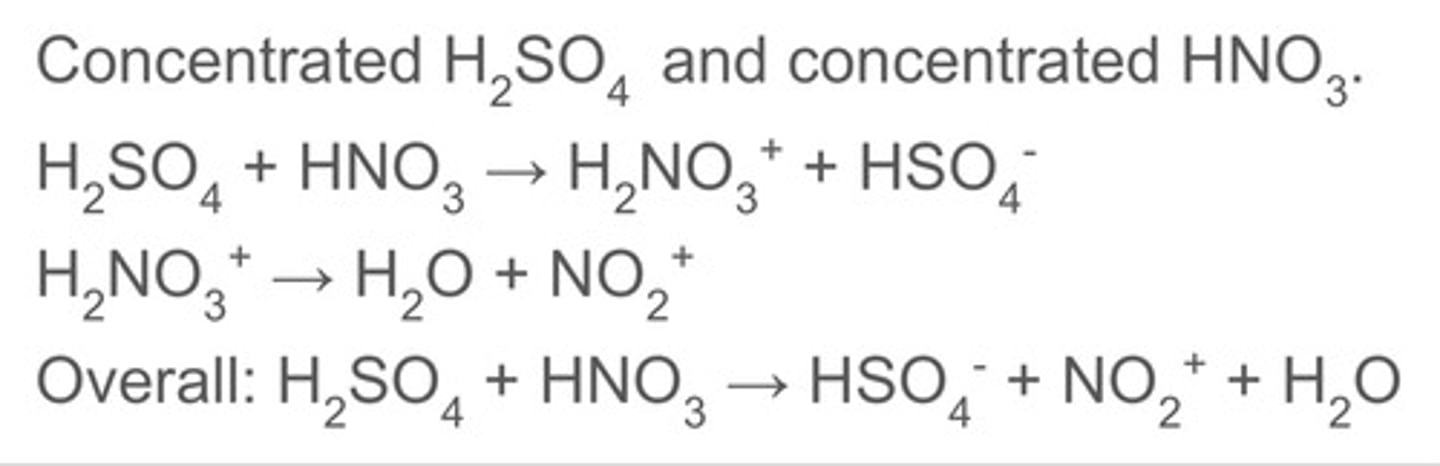
how is the NO2+ ion generated?
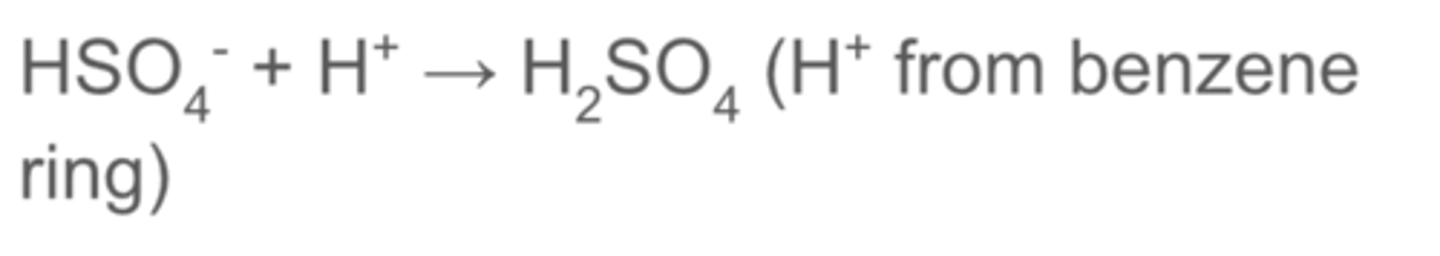
how is the H2SO4 catalyst regenerated in the nitration of benzene?
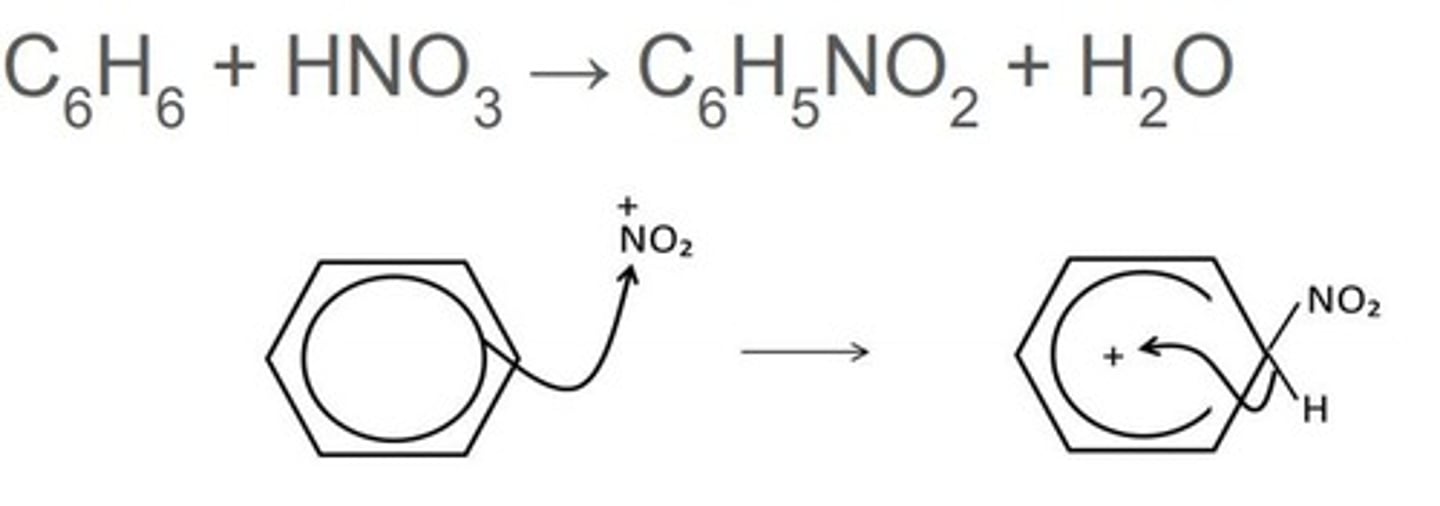
draw a mechanism and write an overall equation for the nitration of benzene
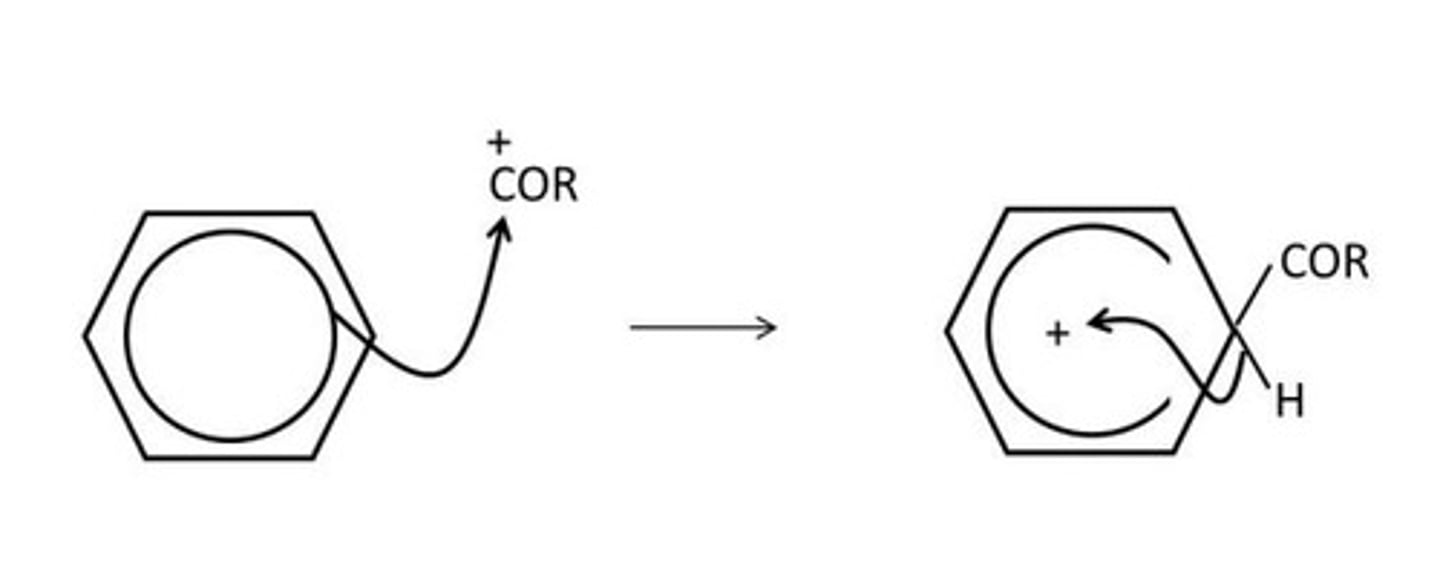
draw the mechanism for the acylation of benzene from RCO+
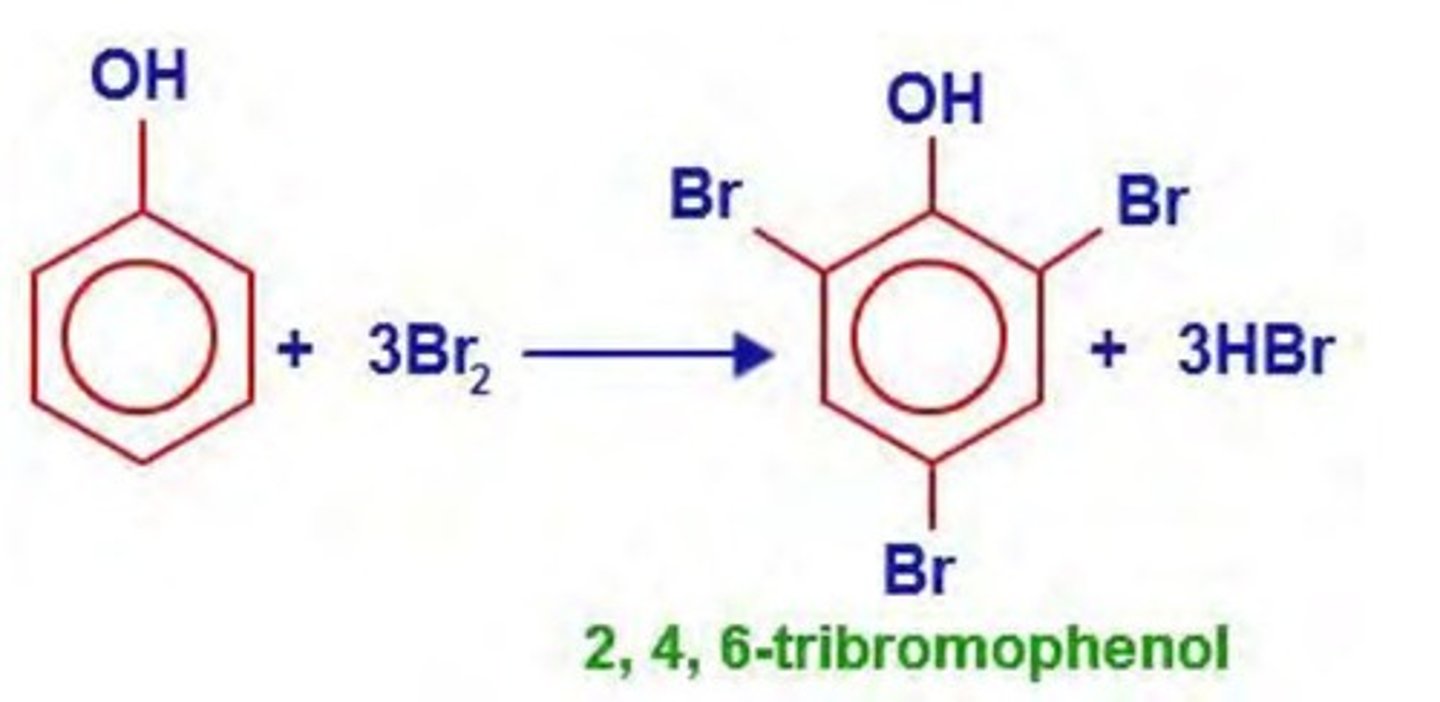
write the equation of the reaction between phenol and bromine to form 2,4,6-tribromophenol
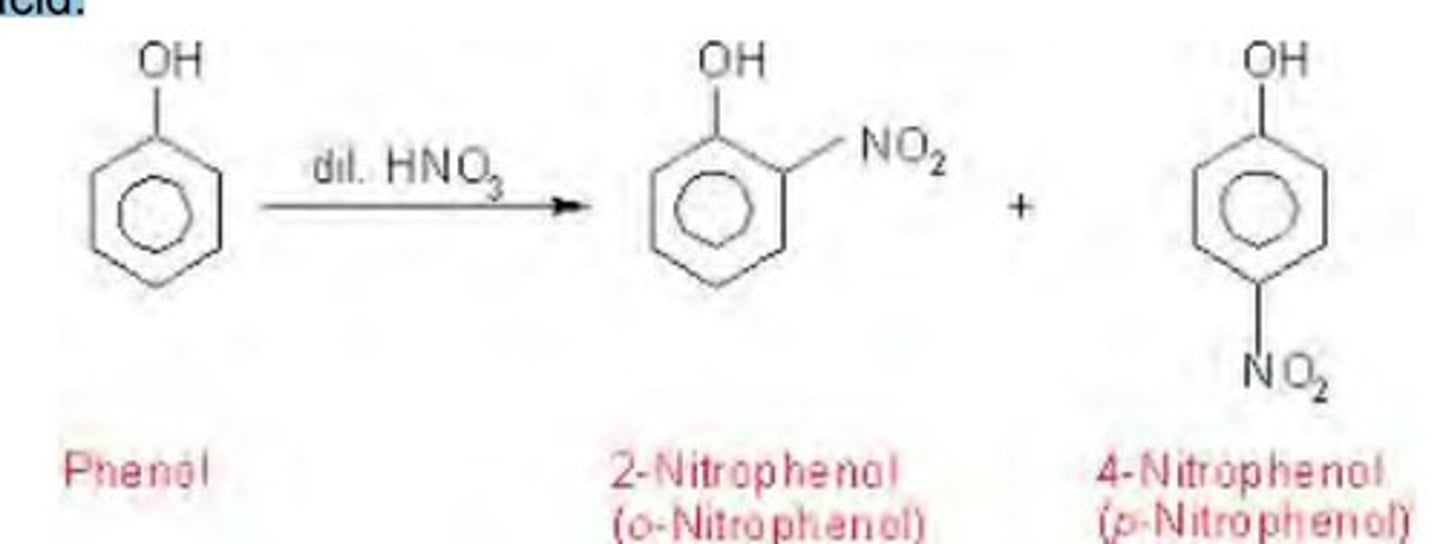
write the equation for the reaction between phenol with dilute nitric acid
draw a mechanism for the nucleophilic addition of a carbonyl compound using Nu to represent the nucleophile
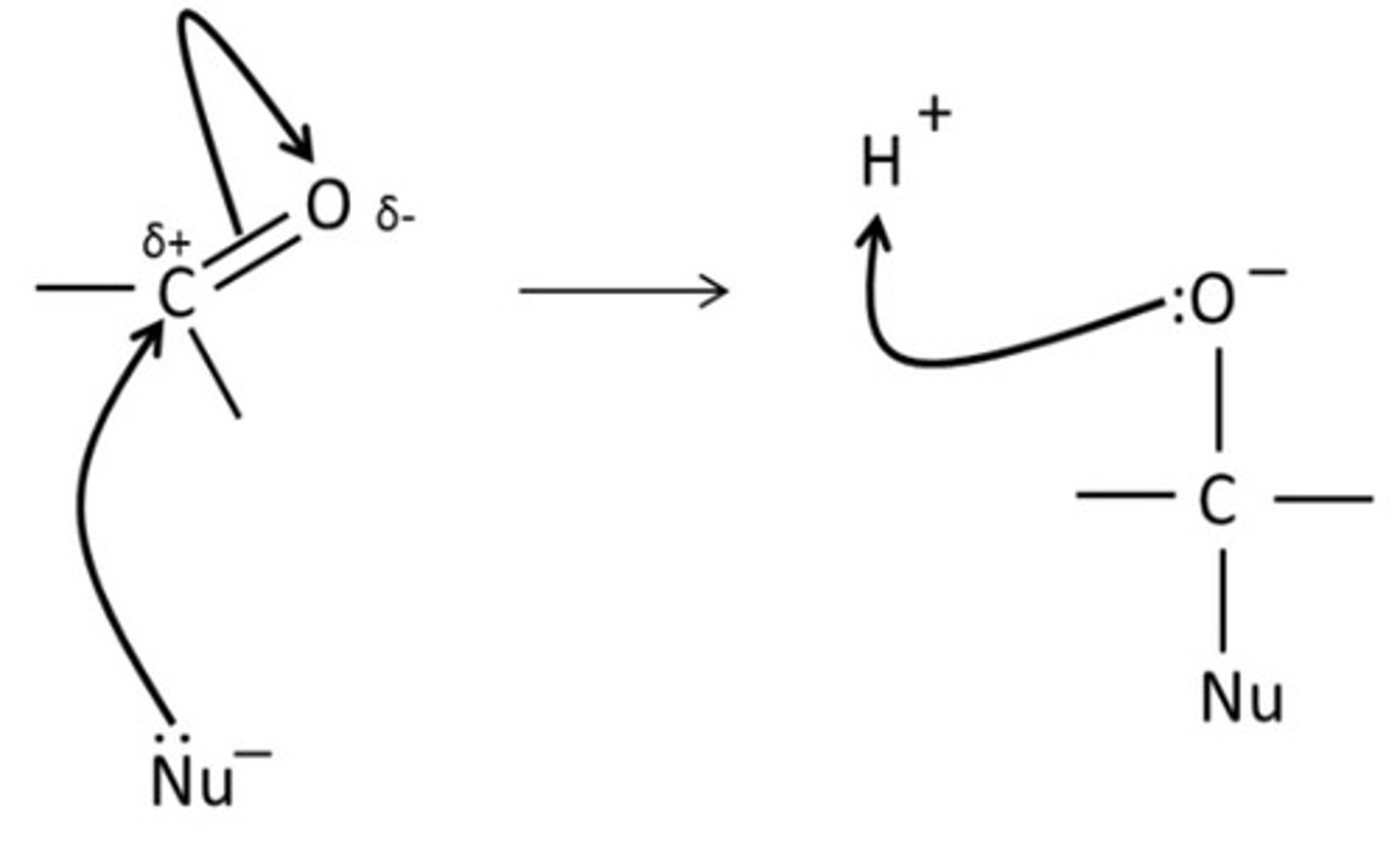
draw a mechanism for nucleophilic addition of HCN to a carbonyl compound
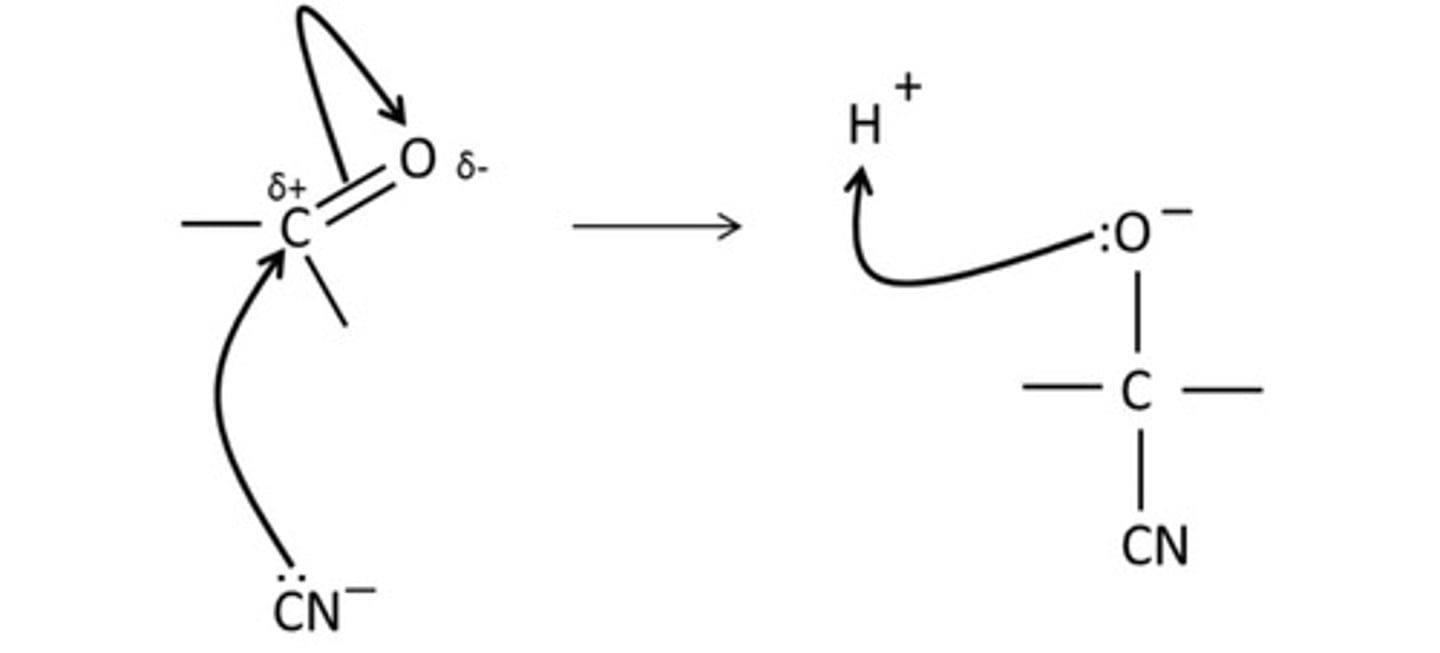
draw the mechanism for the acylation of a nucleophile by an acid derivative
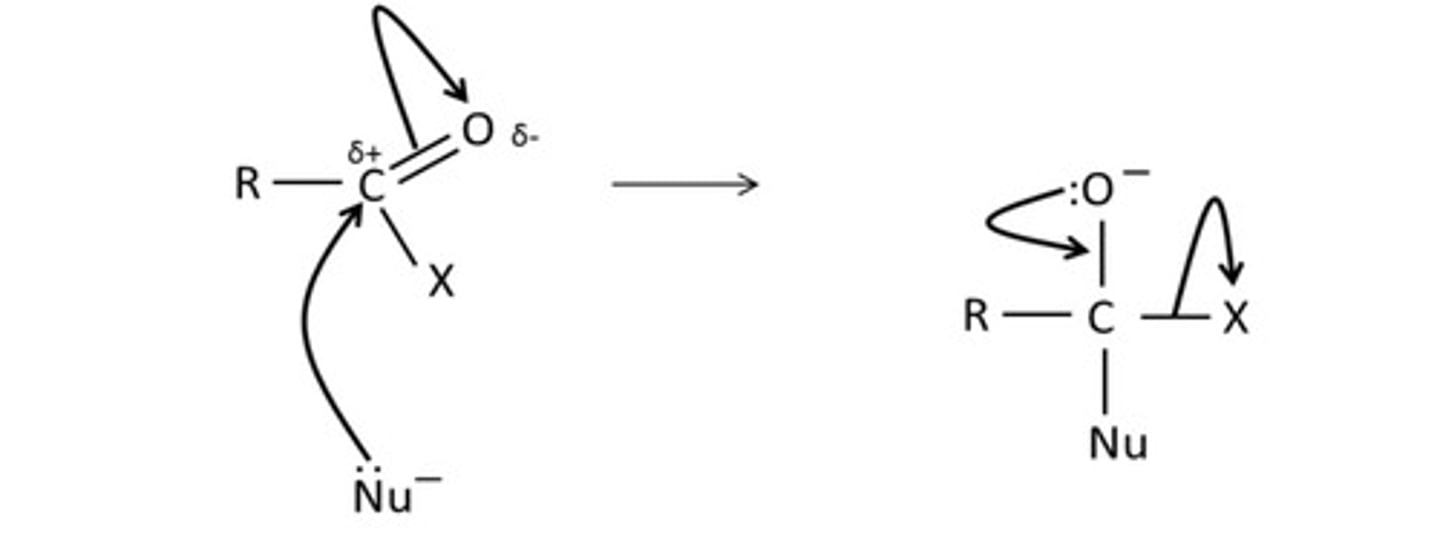
draw the mechanism for the reaction of ethanoyl chloride and ammonia
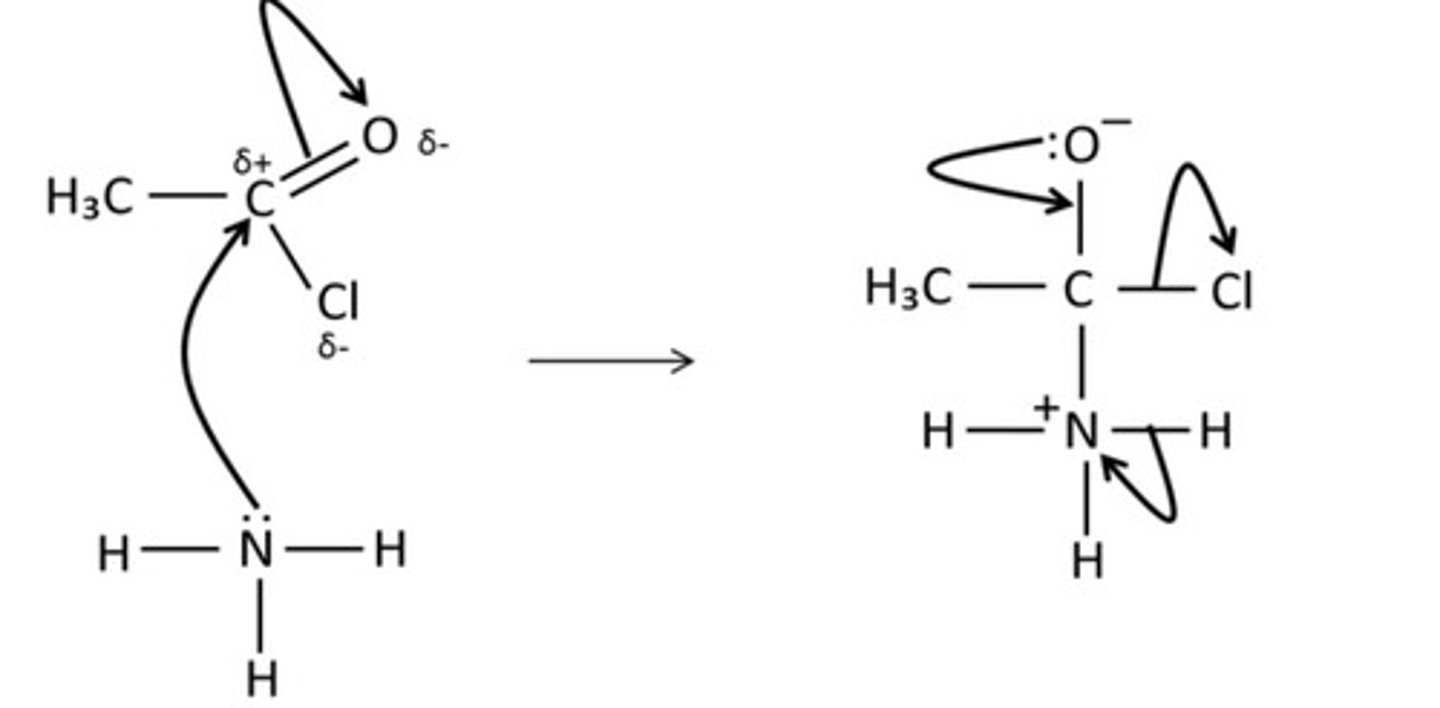
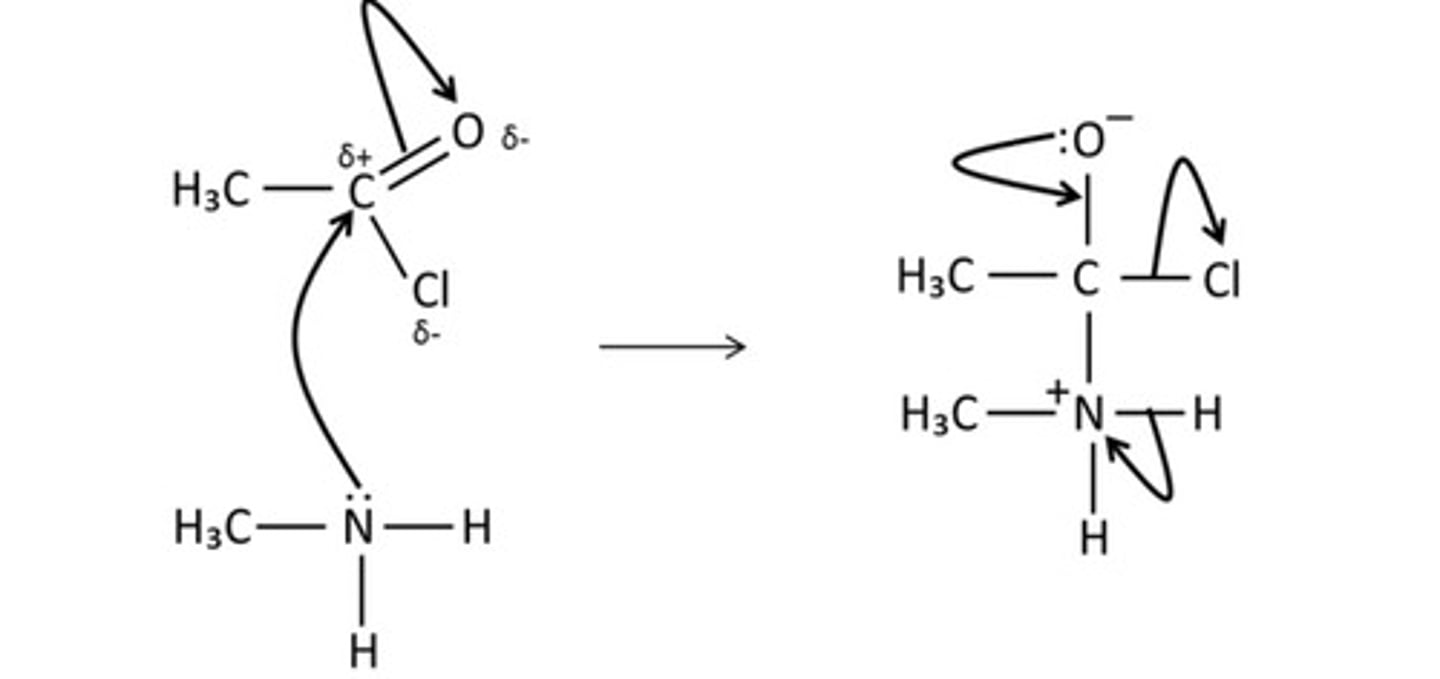
draw the mechanism for the reaction of ethanoyl chloride and methylamine
draw the mechanism for the reaction of ethanoyl chloride and ethanol
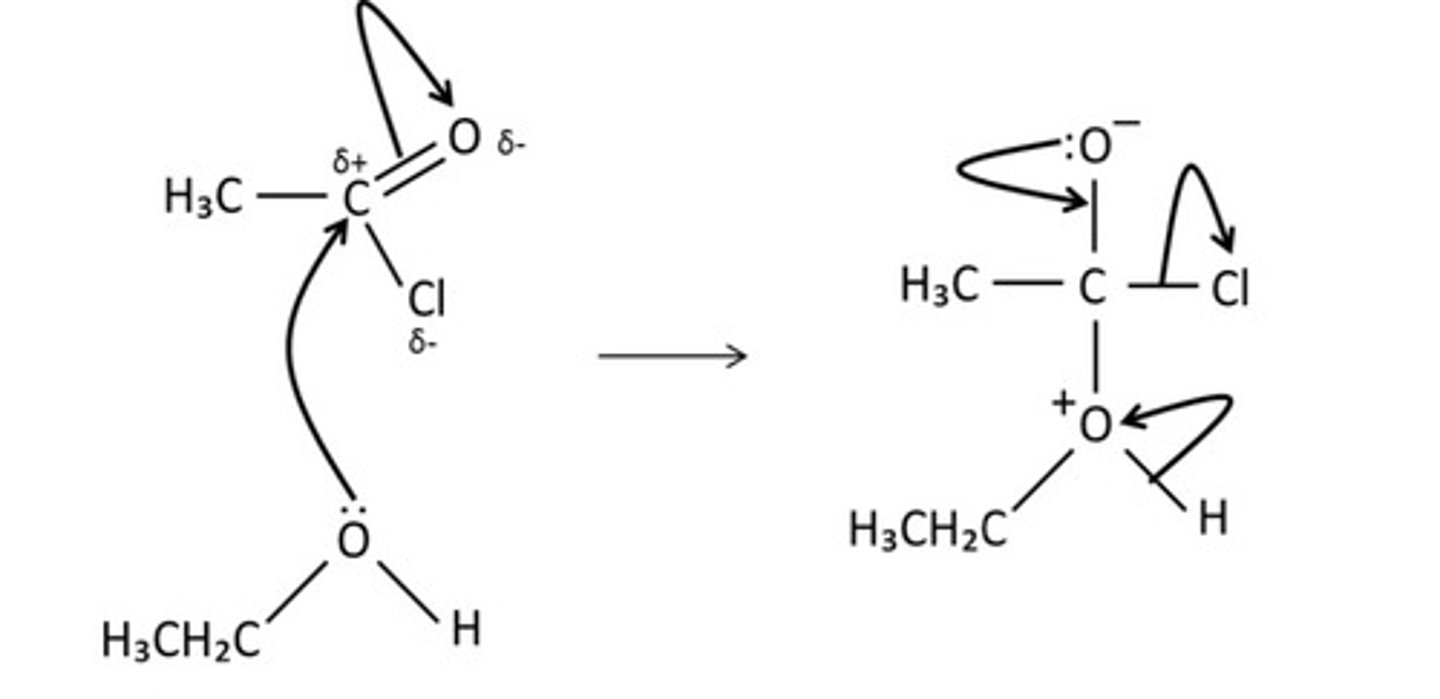
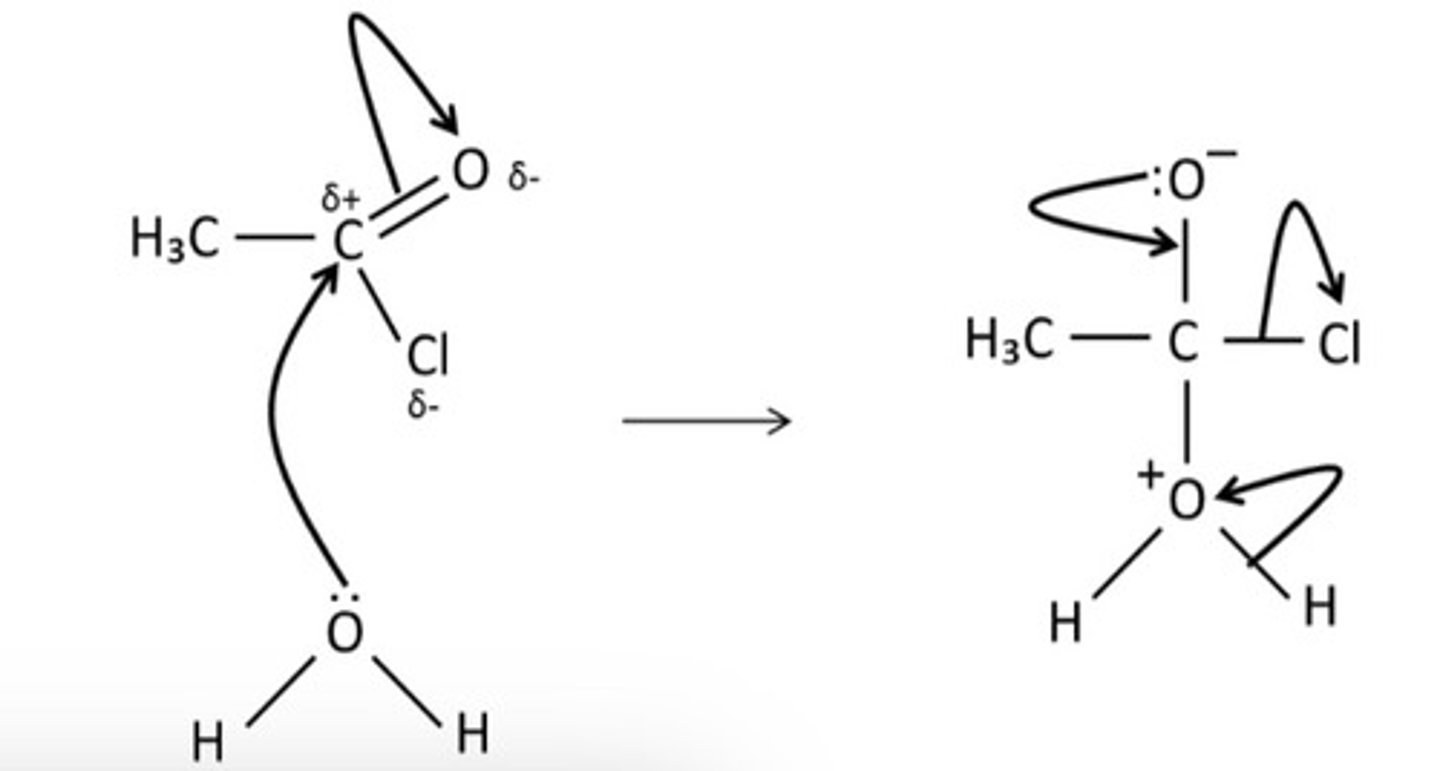
draw the mechanism for the reaction of ethanoyl chloride and water